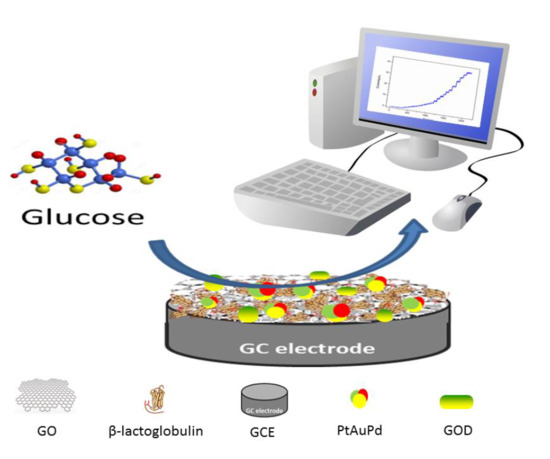
Facile Synthesis of β-Lactoglobulin-Functionalized Reduced Graphene Oxide and Trimetallic PtAuPd Nanocomposite for Electrochemical Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus and Measurements
2.3. Electrode Pre-Treatment
2.4. Preparation of the Modified Sensing Electrodes
3. Results and Discussion
3.1. Characterization of BLG-PtAuPd-RGO
3.2. Electrochemical Performance of BLG-PtAuPd-RGO/GCE
3.3. Electrocatalytic Activity of GOD-BLG-PtAuPd-RGO/GCE
3.4. Chronoamperometric Responses of GOD-BLG-PtAuPd-RGO/GCE
3.5. Stability and Reproducibility of the GOD-BLG-PtAuPd-RGO/GCE Fabricated Glucose Biosensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Mishnaevsky, L. Graphene reinforced nanocomposites: 3D simulation of damage and fracture. Comput. Mater. Sci. 2014, 95, 684–692. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Gao, C. General approach to individually dispersed, highly soluble, and conductive graphene nanosheets functionalized by nitrene chemistry. Chem. Mater. 2010, 22, 5054–5064. [Google Scholar] [CrossRef]
- Goh, M.S.; Pumera, M. Single-, few-, and multilayer graphene not exhibiting significant advantages over graphite microparticles in electroanalysis. Anal. Chem. 2010, 82, 8367–8370. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhu, T.; Zhou, X.; Zhang, Y.; Lou, X.W.; Chen, X.; Zhang, H.; Hng, H.H.; Yan, Q. Facile synthesis of metal oxide/reduced graphene oxide hybrids with high lithium storage capacity and stable cyclability. Nanoscale 2011, 3, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.; Wang, X. Green synthesis of graphene oxide sheets decorated by silver nanoprisms and their anti-bacterial properties. J. Inorg. Biochem. 2011, 105, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.; Okata, T.; Akita, T.; Kohyama, M.; Nakamura, J.; Honma, I. Enhanced electrocatalytic activity of Pt subnanoclusters on graphene nanosheet surface. Nano Lett. 2009, 9, 2255–2259. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.R.; Cristina, G.N.; Kannan, B.; Marko, B.; Klaus, K. Electrochemical modification of graphene. Adv. Mater. 2008, 20, 3050–3053. [Google Scholar]
- Łuczak, T. Comparison of electrochemical oxidation of epinephrine in the presence of interfering ascorbic and uric acids on gold electrodes modified with s-functionalized compounds and gold nanoparticles. Electrochim. Acta 2009, 54, 5863–5870. [Google Scholar] [CrossRef]
- Luque, G.L.; Ferreyra, N.F.; Granero, A.; Bollo, S.; Rivas, G.A. Electrooxidation of DNA at glassy carbon electrodes modified with multiwall carbon nanotubes dispersed in polyethylenimine. Electrochim. Acta 2011, 56, 9121–9126. [Google Scholar] [CrossRef]
- Yue, R.; Shan, L.; Yang, X.; Zhang, W. Approaches to target profiling of natural products. Curr. Med. Chem. 2012, 19, 3841–3855. [Google Scholar] [CrossRef] [PubMed]
- Hirano, I.; Imaoka, T.; Yamamoto, K. Deposition of the monodispersed pt nanodots on a substrate by using the pt nanoparticle-containing dendrimer micelle aqueous solution. J. Inorg. Organomet. Polym. Mater. 2014, 24, 214–218. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, X.; Shen, J.; Zhu, J.-J.; Hou, W. Fabrication of a novel glucose biosensor based on a highly electroactive polystyrene/polyaniline/au nanocomposite. J. Phys. Chem. B 2008, 112, 9237–9242. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, F.; Wang, K.; Han, D.; Zhang, Q.; Yuan, J.; Niu, L. One-step synthesis of graphene–AuNPs by HMTA and the electrocatalytical application for O2 and H2O2. Talanta 2012, 93, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-F.; Zhang, X.-M.; Yao, Z.-J.; Yu, R.; Huang, F.; Wei, X.-W. Enhanced chemiluminescence of the rhodamine 6G−Cerium(IV) system by Au−Ag alloy nanoparticles. J. Phys. Chem. C 2009, 113, 15586–15592. [Google Scholar] [CrossRef]
- Kariuki, N.N.; Khudhayer, W.J.; Karabacak, T.; Myers, D.J. Glad Pt–Ni alloy nanorods for oxygen reduction reaction. ACS Catal. 2013, 3, 3123–3132. [Google Scholar] [CrossRef]
- Han, Y.-F.; Zhong, Z.; Ramesh, K.; Chen, F.; Chen, L.; White, T.; Tay, Q.; Yaakub, S.N.; Wang, Z. Au promotional effects on the synthesis of H2O2 directly from H2 and O2 on supported Pd−Au alloy catalysts. J. Phys. Chem. C 2007, 111, 8410–8413. [Google Scholar] [CrossRef]
- Tan, X.; Prabhudev, S.; Kohandehghan, A.; Karpuzov, D.; Botton, G.A.; Mitlin, D. Pt–Au–Co alloy electrocatalysts demonstrating enhanced activity and durability toward the oxygen reduction reaction. ACS Catal. 2015, 5, 1513–1524. [Google Scholar] [CrossRef]
- Mercer, M.P.; Plana, D.; Fermίn, D.J.; Morgan, D.; Vasiljevic, N. Growth of epitaxial Pt1−xPbx alloys by surface limited redox replacement and study of their adsorption properties. Langmuir 2015, 31, 10904–10912. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Hariharan, S.; Tiwari, A.K. Pt–Ni subsurface alloy catalysts: An improved performance toward CH4 dissociation. J. Phys. Chem. C 2018, 122, 10857–10870. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Kim, J.Y.; Thambidurai, C.; Stickney, J.L. Pb deposition on I-coated Au(111). UHV-EC and EC-STM studies. Langmuir 2007, 23, 2539–2545. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, X.; Xu, Z. Molecular dynamics simulation of the melting behavior of Pt−Au nanoparticles with core−shell structure. J. Phys. Chem. C 2008, 112, 4937–4947. [Google Scholar] [CrossRef]
- Sun, J.; Morales-Lara, F.; Klechikov, A.; Talyzin, A.V.; Baburin, I.A.; Seifert, G.; Cardano, F.; Baldrighi, M.; Frasconi, M.; Giordani, S. Porous graphite oxide pillared with tetrapod-shaped molecules. Carbon 2017, 120, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Hung, W.-S.; Tsou, C.-H.; De Guzman, M.; An, Q.-F.; Liu, Y.-L.; Zhang, Y.-M.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Cross-linking with diamine monomers to prepare composite graphene oxide-framework membranes with varying d-spacing. Chem. Mater. 2014, 26, 2983–2990. [Google Scholar] [CrossRef]
- Burress, J.W.; Gadipelli, S.; Ford, J.; Simmons, J.M.; Zhou, W.; Yildirim, T. Graphene oxide framework materials: Theoretical predictions and experimental results. Angew. Chem. Int. Ed. Engl. 2010, 49, 8902–8904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Y.; Miao, Z.; Ma, M.; Du, X.; Lin, J.; Han, B.; Takahashi, S.; Anzai, J.I.; Chen, Q. Dual-function amperometric sensors based on poly(diallydimethylammoniun chloride)-functionalized reduced graphene oxide/manganese dioxide/gold nanoparticles nanocomposite. Sens. Actuators B Chem. 2016, 222, 663–673. [Google Scholar] [CrossRef]
- Zhang, Z.; Yin, L. Polyvinyl pyrrolidone wrapped Sn nanoparticles/carbon Xerogel composite as anode material for high performance lithium ion batteries. Electrochim. Acta 2016, 212, 594–602. [Google Scholar] [CrossRef]
- Sahihi, M.; Ghayeb, Y.; Bordbar, A.K. Interaction of β-lactoglobulin with resveratrol: Molecular docking and molecular dynamics simulation studies. Chem. Biochem. Eng. Q. 2013, 27, 417–422. [Google Scholar]
- Fragneto, G.; Su, T.J.; Lu, J.R.; Thomas, R.K.; Rennie, A.R. Adsorption of proteins from aqueous solutions on hydrophobic surfaces studied by neutron reflection. Phys. Chem. Chem. Phys. 2000, 2, 5214–5221. [Google Scholar] [CrossRef]
- Feng, X.; Hu, J.; Chen, X.; Xie, J.; Liu, Y. Synthesis and electron transfer property of sulfhydryl-containing multi-walled carbon nanotube/gold nanoparticle heterojunctions. J. Phys. D Appl. Phys. 2009, 42, 042001. [Google Scholar] [CrossRef] [Green Version]
- Hwang, D.-W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Sapountzi, E.; Braiek, M.; Vocanson, F.; Chateaux, J.-F.; Jaffrezic-Renault, N.; Lagarde, F. Gold nanoparticles assembly on electrospun poly(vinyl alcohol)/poly(ethyleneimine)/glucose oxidase nanofibers for ultrasensitive electrochemical glucose biosensing. Sens. Actuators B Chem. 2017, 238, 392–401. [Google Scholar] [CrossRef]
- Uehara, H.; Kakiage, M.; Sekiya, M.; Sakuma, D.; Yamonobe, T.; Takano, N.; Barraud, A.; Meurville, E.; Ryser, P. Size-selective diffusion in nanoporous but flexible membranes for glucose sensors. ACS Nano 2009, 3, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Kwon, D.; Lee, D.; Lee, T.H.; Lee, J.H.; Lee, G.; Yoon, D.S. A highly permselective electrochemical glucose sensor using red blood cell membrane. Biosens. Bioelectron. 2018, 102, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Eguílaz, M.; Villalonga, R.; Pingarrón, J.M.; Ferreyra, N.F.; Rivas, G.A. Functionalization of bamboo-like carbon nanotubes with 3-mercaptophenylboronic acid-modified gold nanoparticles for the development of a hybrid glucose enzyme electrochemical biosensor. Sens. Actuators B Chem. 2015, 216, 629–637. [Google Scholar] [CrossRef]
- Rocha, D.L.; Rocha, F.R.P. A flow-based procedure with solenoid micro-pumps for the spectrophotometric determination of uric acid in urine. Microchem. J. 2010, 94, 53–59. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Wang, Z.H.; Wang, H.; Zhao, R.; Ding, M.Y. Determination of uric acid in human urine by ion chromatography with conductivity detector. Chin. Chem. Lett. 2011, 22, 342–345. [Google Scholar] [CrossRef]
- Wu, F.; Huang, Y.; Li, Q. Animal tissue-based chemiluminescence sensing of uric acid. Anal. Chim. Acta 2005, 536, 107–113. [Google Scholar] [CrossRef]
- Galbán, J.; Andreu, Y.; Almenara, M.J.; de Marcos, S.; Castillo, J.R. Direct determination of uric acid in serum by a fluorometric-enzymatic method based on uricase. Talanta 2001, 54, 847–854. [Google Scholar] [CrossRef]
- Perelló, J.; Sanchis, P.; Grases, F. Determination of uric acid in urine, saliva and calcium oxalate renal calculi by high-performance liquid chromatography/mass spectrometry. J. Chromatogr. B 2005, 824, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Miao, Z.; Zhang, D.; Fang, Y.; Ma, M.; Chen, Q. Facile synthesis of beta-lactoglobulin-functionalized multi-wall carbon nanotubes and gold nanoparticles on glassy carbon electrode for electrochemical sensing. Biosens. Bioelectron. 2014, 62, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Ngamchuea, K.; Eloul, S.; Tschulik, K.; Compton, R.G. Planar diffusion to macro disc electrodes—What electrode size is required for the Cottrell and Randles-Sevcik equations to apply quantitatively? J. Solid State Electrochem. 2014, 18, 3251–3257. [Google Scholar] [CrossRef]
- Zhou, X.; Dai, X.; Li, J.; Long, Y.; Li, W.; Tu, Y. A sensitive glucose biosensor based on Ag@C core–shell matrix. Mater. Sci. Eng. C 2015, 49, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Wang, P.; Zhong, A.; Yang, M.; Xu, Q.; Hao, S.; Hu, X. Development of a glucose biosensor based on electrodeposited gold nanoparticles-polyvinylpyrrolidone-polyaniline nanocomposites. J. Electroanal. Chem. 2015, 756, 153–160. [Google Scholar] [CrossRef]
- Yang, Z.; Tang, Y.; Li, J.; Zhang, Y.; Hu, X. Facile synthesis of tetragonal columnar-shaped Tio2 nanorods for the construction of sensitive electrochemical glucose biosensor. Biosens. Bioelectron. 2014, 54, 528–533. [Google Scholar] [CrossRef] [PubMed]








| Electrode Composite Materials | Linearity (mM) | Detection Limit (μM) | Sensitivity (μA mM−1 cm−2) | pH | Reference |
|---|---|---|---|---|---|
| GOD−Ag@C/Nafion/GCE | 0.05–2.5 | 20 | 24.65 | 7.0 | [43] |
| GOx-AuNPs-PVP a-PANI b/GCE | 0.05–2.25 | 10 | 9.62 | 7.0 | [44] |
| 4ATP c/PVA d/PEI e/AuNPs/GOx/AuE | 0.01–0.2 | 0.9 | - | 5.0 | [32] |
| GOx/TCS−TiO2 f/chitosan/GCE | 0.005–1.32 | 2.0 | 23.2 | 7.0 | [45] |
| bMWCNTs-HBPEI g/AuNPs-B(OH)2/GOx/GCE | 0.25–5 | 0.8 | 28.6 | 7.4 | [35] |
| GOD/BLG/multi wall carbon nanotubes/gold nanoparticles/GCE | 0.025–5.5 | 1.1 | 56.3 | 7.0 | [41] |
| GOD-BLG-PtAuPd-RGO/GCE | 0.005–9 | 0.13 | 63.29 | 7.0 | This work |
| Sample | Calculated by Local Hospital (mM) | Detected by Fabricated Sensor (mM) | Glucose Added (mM) | Glucose Found (mM) | Recovery (%) | RSD (%, n = 5) |
|---|---|---|---|---|---|---|
| 1 | 4.20 | 4.19 | 0.2 | 4.38 | 95.00 | 2.79 |
| 2 | 3.62 | 3.64 | 0.4 | 4.03 | 97.50 | 3.37 |
| 3 | 3.91 | 3.92 | 0.6 | 4.53 | 101.67 | 2.45 |
| 4 | 4.17 | 4.20 | 0.8 | 5.12 | 102.50 | 2.89 |
| 5 | 4.58 | 4.56 | 1.0 | 5.52 | 96.00 | 3.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, B.; Pan, M.; Zhou, J.; Wang, Y.; Wang, Z.; Jiao, J.; Zhang, C.; Chen, Q. Facile Synthesis of β-Lactoglobulin-Functionalized Reduced Graphene Oxide and Trimetallic PtAuPd Nanocomposite for Electrochemical Sensing. Nanomaterials 2018, 8, 724. https://doi.org/10.3390/nano8090724
Han B, Pan M, Zhou J, Wang Y, Wang Z, Jiao J, Zhang C, Chen Q. Facile Synthesis of β-Lactoglobulin-Functionalized Reduced Graphene Oxide and Trimetallic PtAuPd Nanocomposite for Electrochemical Sensing. Nanomaterials. 2018; 8(9):724. https://doi.org/10.3390/nano8090724
Chicago/Turabian StyleHan, Bingkai, Meixin Pan, Jiexin Zhou, Yingying Wang, Zihua Wang, Jun Jiao, Cong Zhang, and Qiang Chen. 2018. "Facile Synthesis of β-Lactoglobulin-Functionalized Reduced Graphene Oxide and Trimetallic PtAuPd Nanocomposite for Electrochemical Sensing" Nanomaterials 8, no. 9: 724. https://doi.org/10.3390/nano8090724
APA StyleHan, B., Pan, M., Zhou, J., Wang, Y., Wang, Z., Jiao, J., Zhang, C., & Chen, Q. (2018). Facile Synthesis of β-Lactoglobulin-Functionalized Reduced Graphene Oxide and Trimetallic PtAuPd Nanocomposite for Electrochemical Sensing. Nanomaterials, 8(9), 724. https://doi.org/10.3390/nano8090724