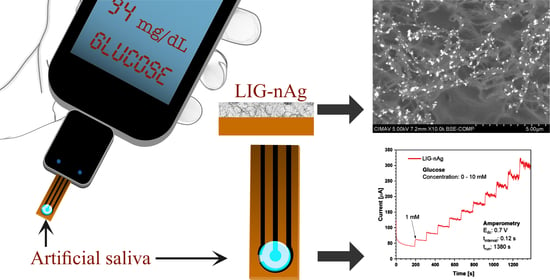
Salivary Glucose Detection with Laser Induced Graphene/AgNPs Non-Enzymatic Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment and Material Characterization
2.3. LIG Electrodes Design and Manufacture
2.4. Non-Enzymatic Electrochemical Glucose Detection
2.5. Selectivity and Real Sample Evaluation
3. Results and Discussion
3.1. Material Characterization
3.2. Exploratory Analysis in CV for Glucose Detection
3.3. Amperometric Detection of Glucose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klatman, E.L.; Jenkins, A.J.; Ahmedani, M.Y.; Ogle, G.D. Blood Glucose Meters and Test Strips: Global Market and Challenges to Access in Low-Resource Settings. Lancet Diabetes Endocrinol. 2019, 7, 150–160. [Google Scholar] [CrossRef] [PubMed]
- IDF. IDF Diabetes Atlas, 10th ed.; IDF: Brussels, Belgium, 2021; ISBN 9782930229980. [Google Scholar]
- Nery, E.W.; Kundys, M.; Jeleń, P.S.; Jönsson-Niedziólka, M. Electrochemical Glucose Sensing: Is There Still Room for Improvement? Anal. Chem. 2016, 88, 11271–11282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tee, S.Y.; Teng, C.P.; Ye, E. Metal Nanostructures for Non-Enzymatic Glucose Sensing. Mater. Sci. Eng. C 2017, 70, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.H.; Vyas, C.; Grieve, B.; Bartolo, P. Recent Advances in Enzymatic and Non-Enzymatic Electrochemical Glucose Sensing. Sensors 2021, 21, 4672. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Qiao, Y.; Zhao, H.; Liang, J.; Li, T.; Luo, Y.; Lu, S.; Xifeng, S.; Lu, W.; Sun, X. Electrochemical Non-Enzymatic Glucose Sensors: Recent Progress and Perspectives. Chem. Commun. 2020, 56, 14553–14569. [Google Scholar] [CrossRef] [PubMed]
- Justice Babu, K.; Sheet, S.; Lee, Y.S.; Gnana Kumar, G. Three-Dimensional Dendrite Cu-Co/Reduced Graphene Oxide Architectures on a Disposable Pencil Graphite Electrode as an Electrochemical Sensor for Nonenzymatic Glucose Detection. ACS Sustain. Chem. Eng. 2018, 6, 1909–1918. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, J.; Tang, P.; Morante, J.R.; Arbiol, J.; Xu, C.; Li, Q.; Fransaer, J. Ultrasensitive Binder-Free Glucose Sensors Based on the Pyrolysis of in Situ Grown Cu MOF. Sens. Actuators B Chem. 2018, 254, 272–281. [Google Scholar] [CrossRef] [Green Version]
- Chitare, Y.M.; Jadhav, S.B.; Pawaskar, P.N.; Magdum, V.v.; Gunjakar, J.L.; Lokhande, C.D. Metal Oxide-Based Composites in Nonenzymatic Electrochemical Glucose Sensors. Ind. Eng. Chem. Res. 2021, 60, 18195–18217. [Google Scholar] [CrossRef]
- Zhuang, X.; Tian, C.; Luan, F.; Wu, X.; Chen, L. One-step electrochemical fabrication of a nickel oxide nanoparticle/polyaniline nanowire/graphene oxide hybrid on a glassy carbon electrode for use as a non-enzymatic glucose biosensor. RSC Adv. 2016, 6, 92541–92546. [Google Scholar] [CrossRef]
- Dilmac, Y.; Guler, M. Fabrication of Non-Enzymatic Glucose Sensor Dependent upon Au Nanoparticles Deposited on Carboxylated Graphene Oxide. J. Electroanal. Chem. 2020, 864, 114091. [Google Scholar] [CrossRef]
- Dayakar, T.; Rao, K.V.; Bikshalu, K.; Malapati, V.; Sadasivuni, K.K. Non-Enzymatic Sensing of Glucose Using Screen-Printed Electrode Modified with Novel Synthesized CeO2@CuO Core Shell Nanostructure. Biosens. Bioelectron. 2018, 111, 166–173. [Google Scholar] [CrossRef]
- Raza, W.; Ahmad, K. A Highly Selective Fe@ZnO Modified Disposable Screen Printed Electrode Based Non-Enzymatic Glucose Sensor (SPE/Fe@ZnO). Mater. Lett. 2018, 212, 231–234. [Google Scholar] [CrossRef]
- Hernández-Saravia, L.P.; Martinez, T.; Llanos, J.; Bertotti, M. A Cu-NPG/SPE Sensor for Non-Enzymatic and Non-Invasive Electrochemical Glucose Detection. Microchem. J. 2021, 160, 105629. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, G.; Yao, A.; Xiao, Y.; Du, J.; Guo, Y.; Xiao, D.; Hu, Q.; Choi, M.M.F. A Sensitive AgNPs/CuO Nanofibers Non-Enzymatic Glucose Sensor Based on Electrospinning Technology. Sens. Actuators B Chem. 2014, 195, 431–438. [Google Scholar] [CrossRef]
- Sajadpour, M.; Siampour, H.; Abbasian, S.; Amiri, M.; Rameshan, R.; Rameshan, C.; Hajian, A.; Bagheri, H.; Moshaii, A. A Non-Enzymatic Glucose Sensor Based on the Hybrid Thin Films of Cu on Acetanilide/ITO. J. ElectroChem. Soc. 2019, 166, B1116–B1125. [Google Scholar] [CrossRef]
- Khoshroo, A.; Sadrjavadi, K.; Taran, M.; Fattahi, A. Electrochemical System Designed on a Copper Tape Platform as a Nonenzymatic Glucose Sensor. Sens. Actuators B Chem. 2020, 325, 128778. [Google Scholar] [CrossRef]
- Rani, S.D.; Ramachandran, R.; Sheet, S.; Aziz, M.A.; Lee, Y.S.; Al-Sehemi, A.G.; Pannipara, M.; Xia, Y.; Tsai, S.Y.; Ng, F.L.; et al. NiMoO4 Nanoparticles Decorated Carbon Nanofiber Membranes for the Flexible and High Performance Glucose Sensors. Sens. Actuators B Chem. 2020, 312, 127886. [Google Scholar] [CrossRef]
- Ambaye, A.D.; Kefeni, K.K.; Mishra, S.B.; Nxumalo, E.N.; Ntsendwana, B. Recent Developments in Nanotechnology-Based Printing Electrode Systems for Electrochemical Sensors. Talanta 2021, 225, 121951. [Google Scholar] [CrossRef]
- Wang, F.; Wang, K.; Zheng, B.; Dong, X.; Mei, X.; Lv, J.; Duan, W.; Wang, W. Laser-Induced Graphene: Preparation, Functionalization and Applications. Mater. Technol. 2018, 33, 340–356. [Google Scholar] [CrossRef]
- Kaidarova, A.; Kosel, J. Physical Sensors Based on Laser-Induced Graphene: A Review. IEEE Sens. J. 2021, 21, 12426–12443. [Google Scholar] [CrossRef]
- Vivaldi, F.M.; Dallinger, A.; Bonini, A.; Poma, N.; Sembranti, L.; Biagini, D.; Salvo, P.; Greco, F.; di Francesco, F. Three-Dimensional (3D) Laser-Induced Graphene: Structure, Properties, and Application to Chemical Sensing. ACS Appl. Mater. Interfaces 2021, 13, 30245–30260. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ji, H.; Lv, X.; Zeng, C.; Li, H.; Li, F.; Qu, B.; Cui, F.; Zhou, Q. Laser-Induced Graphene (LIG)-Driven Medical Sensors for Health Monitoring and Diseases Diagnosis. Microchim. Acta 2022, 189, 54. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.T.S.G.; Souto, D.E.P.; Barragan, J.T.C.; de Fatima Giarola, J.; de Moraes, A.C.M.; Kubota, L.T. Electrochemical Biosensors in Point-of-Care Devices: Recent Advances and Future Trends. ChemElectroChem 2017, 4, 778–794. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Xiang, Y.; Wang, D.; Zhang, P.; Wang, Y.; Lu, S.; Xu, R.; Zhao, J. A Flexible Non-Enzymatic Glucose Sensor Based on Copper Nanoparticles Anchored on Laser-Induced Graphene. Carbon NY 2020, 156, 506–513. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, S.; Hu, Z.; Zhang, X.; Yi, N.; Tang, K.; Dexheimer, M.G.; Lian, X.; Wang, Q.; Yang, J.; et al. Laser-Induced Graphene Non-Enzymatic Glucose Sensors for on-Body Measurements. Biosens. Bioelectron. 2021, 193, 113606. [Google Scholar] [CrossRef]
- Zahed, M.A.; Barman, S.C.; Das, P.S.; Sharifuzzaman, M.; Yoon, H.S.; Yoon, S.H.; Park, J.Y. Highly Flexible and Conductive Poly (3,4-Ethylene Dioxythiophene)-Poly (Styrene Sulfonate) Anchored 3-Dimensional Porous Graphene Network-Based Electrochemical Biosensor for Glucose and PH Detection in Human Perspiration. Biosens. Bioelectron. 2020, 160, 112220. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, X.; Song, W. Physical and Chemical Sensors on the Basis of Laser-Induced Graphene: Mechanisms, Applications, and Perspectives. ACS Nano 2021, 15, 18708–18741. [Google Scholar] [CrossRef]
- Darabdhara, G.; Das, M.R.; Singh, S.P.; Rengan, A.K.; Szunerits, S.; Boukherroub, R. Historical Perspective Ag and Au Nanoparticles/Reduced Graphene Oxide Composite Materials: Synthesis and Application in Diagnostics and Therapeutics. Adv. Colloid Interface Sci. 2019, 271, 101991. [Google Scholar] [CrossRef]
- Sharma, S.; Prakash, V.; Mehta, S.K. Graphene/Silver Nanocomposites-Potential Electron Mediators for Proliferation in Electrochemical Sensing and SERS Activity. TrAC Trends Anal. Chem. 2017, 86, 155–171. [Google Scholar] [CrossRef]
- Shabbir, S.A.; Tariq, S.; Gul Bahar Ashiq, M.; Khan, W.A. Non-Enzymatic Glucose Sensor with Electrodeposited Silver/Carbon Nanotubes Composite Electrode. Biosci. Rep. 2019, 39, 1–13. [Google Scholar] [CrossRef]
- Hill-Dick, J.O.; Harmon, A.; Cao, W.; Sadasivuni, K.K.; Elahi, M.; Adedeji, A.; Elsayed-Ali, H.E.; Slaughter, G.; Kumar, K.; Asadi, M.; et al. Nanocoral Ag for Nonenzymatic Glucose Detection at Extremely Low Operational Potential. Mater. Today Commun. 2021, 27, 102261. [Google Scholar] [CrossRef]
- Uzunoglu, A.; Kose, D.A.; Gokmese, E.; Gokmese, F. Electrochemical Glucose Detection Using PdAg Nanoparticles Anchored on RGO/MWCNT Nanohybrids. J. Clust. Sci. 2020, 31, 231–239. [Google Scholar] [CrossRef]
- Baghayeri, M.; Amiri, A.; Farhadi, S. Development of Non-Enzymatic Glucose Sensor Based on Efficient Loading Ag Nanoparticles on Functionalized Carbon Nanotubes. Sens. Actuators B Chem. 2016, 225, 354–362. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Zandi-Atashbar, N.; Rezaei, B.; Ghiaci, M.; Chermahini, M.E.; Moshiri, P. Non-Enzymatic Glucose Electrochemical Sensor Based on Silver Nanoparticle Decorated Organic Functionalized Multiwall Carbon Nanotubes. RSC Adv. 2016, 6, 60926–60932. [Google Scholar] [CrossRef]
- Luan, V.H.; Han, J.H.; Kang, H.W.; Lee, W. Ultra-sensitive non-enzymatic amperometric glucose sensors based on silver nanowire/graphene hybrid three-dimensional nanostructures. Results Phys. 2019, 15, 102761. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, D.; Zhong, C.; Xiong, R.; Shi, J.; Liu, Z.; Wang, X.; Lei, Q. Surface Morphology and Raman Analysis of the Polyimide Film Aged under Bipolar Pulse Voltage. Polym. Eng. Sci. 2013, 53, 1536–1541. [Google Scholar] [CrossRef]
- Plis, E.A.; Engelhart, D.P.; Cooper, R.; Johnston, W.R.; Ferguson, D.; Hoffmann, R. Review of Radiation-Induced Effects in Polyimide. Appl. Sci. 2019, 9, 1999. [Google Scholar] [CrossRef] [Green Version]
- Shahriary, L.; Athawale, A.A. Electrochemical Deposition of Silver/Silver Oxide on Reduced Graphene Oxide for Glucose Sensing. J. Solid State Electrochem. 2015, 19, 2255–2263. [Google Scholar] [CrossRef]
- Khalaf, N.; Ahamad, T.; Naushad, M.; Al-hokbany, N.; Al-Saeedi, S.I.; Almotairi, S.; Alshehri, S.M. Chitosan Polymer Complex Derived Nanocomposite (AgNPs/NSC) for Electrochemical Non-Enzymatic Glucose Sensor. Int. J. Biol. Macromol. 2020, 146, 763–772. [Google Scholar] [CrossRef]
- Jia, M.; Wang, T.; Liang, F.; Hu, J. A Novel Process for the Fabrication of a Silver-Nanoparticle-Modified Electrode and Its Application in Nonenzymatic Glucose Sensing. Electroanalysis 2012, 24, 1864–1868. [Google Scholar] [CrossRef]
- Droog, J.M.M.; Huisman, F. Electrochemical Formation and Reduction of Silver Oxides in Alkaline Media. J. Electroanal. Chem. 1980, 115, 211–224. [Google Scholar] [CrossRef]
- Islam, S.N.; Naqvi, S.M.A.; Parveen, S.; Ahmad, A. Application of Mycogenic Silver/Silver Oxide Nanoparticles in Electrochemical Glucose Sensing; alongside Their Catalytic and Antimicrobial Activity. 3 Biotech. 2021, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Pasta, M.; la Mantia, F.; Cui, Y. Mechanism of Glucose Electrochemical Oxidation on Gold Surface. Electrochim. Acta 2010, 55, 5561–5568. [Google Scholar] [CrossRef]
- Pasta, M.; Ruffo, R.; Falletta, E.; Mari, C.M.; della Pina, C. Alkaline Glucose Oxidation on Nanostructured Gold Electrodes. Gold Bull. 2010, 43, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Abd El Rehim, S.S.; Hassan, H.H.; Ibrahim, M.A.M.; Amin, M.A. Electrochemical Behaviour of a Silver Electrode in NaOH Solutions. Monatsh. Chem. 1998, 129, 1103–1117. [Google Scholar] [CrossRef]
- Vassilyev, Y.B.; Khazova, O.A.; Nikolaeva, N.N. Kinetics and Mechanism of Glucose Electroxidation on Different Electrode-Catalysts. J. Electroanal. Chem. 1985, 196, 127–144. [Google Scholar] [CrossRef]
- Quan, H.; Park, S.U.; Park, J. Electrochemical Oxidation of Glucose on Silver Nanoparticle-Modified Composite Electrodes. Electrochim. Acta 2010, 55, 2232–2237. [Google Scholar] [CrossRef]









| WE | Sensing Material | Synthesis | Potential [V] | Working Range [𝛍M] | Selectivity | LOD [𝛍M] | Real Sample | Integrated System | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Cu Tape | Ag | galvanic replacement | 0.65 | 3–3300 | -- | 1.1 | serum | X | [17] |
| GCE | AgNPs/ f-MWCNT | chemical reduction | 0.58 | 1.3–1000 | 1057.3 mA/mM | 0.003 | serum | X | [35] |
| GCE | Ag/Ag2O /rGO | electrodeposition | 0.6 | 200–8000 | 32 μA/mM · cm | 0.06 | -- | X | [39] |
| GCE | Ag/NSC/ Nafion | thermal | 0.6 | 5–3000 | -- | 46 | -- | X | [40] |
| LIG | AgNPs-G1 | electrodeposition | 0.7 | 0–10000 | 24.1 μA/mM | 412 | artificial saliva | YES | This work |
| LIG | AgNPs-G2 | electrodeposition | 0.55 | 0–2000 | 52.2 μA/mM | 45 | artificial saliva | YES | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aparicio-Martínez, E.P.; Vega-Rios, A.; Osuna, V.; Dominguez, R.B. Salivary Glucose Detection with Laser Induced Graphene/AgNPs Non-Enzymatic Sensor. Biosensors 2023, 13, 207. https://doi.org/10.3390/bios13020207
Aparicio-Martínez EP, Vega-Rios A, Osuna V, Dominguez RB. Salivary Glucose Detection with Laser Induced Graphene/AgNPs Non-Enzymatic Sensor. Biosensors. 2023; 13(2):207. https://doi.org/10.3390/bios13020207
Chicago/Turabian StyleAparicio-Martínez, Eider Pedro, Alejandro Vega-Rios, Velia Osuna, and Rocio Berenice Dominguez. 2023. "Salivary Glucose Detection with Laser Induced Graphene/AgNPs Non-Enzymatic Sensor" Biosensors 13, no. 2: 207. https://doi.org/10.3390/bios13020207
APA StyleAparicio-Martínez, E. P., Vega-Rios, A., Osuna, V., & Dominguez, R. B. (2023). Salivary Glucose Detection with Laser Induced Graphene/AgNPs Non-Enzymatic Sensor. Biosensors, 13(2), 207. https://doi.org/10.3390/bios13020207