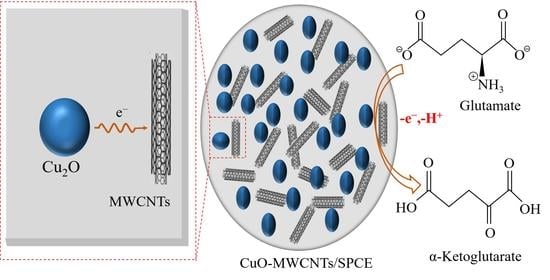
Nonenzymatic Electrochemical Glutamate Sensor Using Copper Oxide Nanomaterials and Multiwall Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Preparation of Samples
2.2. Apparatus
2.3. Synthesis of Copper Oxide Nanostructure
2.4. Preparation of CuO-MWCNTs/SPCE Electrode
3. Results and Discussion
3.1. Characterization of CuO and CuO-MWCNT/SPCE Electrode
3.1.1. Identification of Copper Oxides
3.1.2. Surface Morphology of Fabricated CuO and CuO-MWCNTs/SPCE
3.2. Performance Optimization of Sensing Electrode
3.3. Sensing Performance of the CuO-MWCNTs/SPCE Electrode
3.4. Effect of Scan Rate on Glutamate Sensing
3.5. Effect of pH on Glutamate Sensing
3.6. Voltammetric Determination of Glutamate
3.7. Interference Studies
3.8. Real Sample Analysis
3.9. Effect of Temperature
3.10. Repeatability and Reproducibility Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitt, D.; Werner, P.; Raine, C.S. Glutamate excitotoxicity in a model of multiple sclerosis. Nat. Med. 2000, 6, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, A.; Boykoo, M.; Shapira, Y.; Zlotnik, A. Blood glutamate scavenging: Insight into neuroprotection. Int. J. Mol. Sci. 2012, 13, 10041–10066. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.F.; Linher-Melville, K.; Wu, J.; Fazzari, J.; Miladinovic, T.; Ungard, R.; Zhu, K.L.; Singh, G. Bone cancer-induced pain is associated with glutamate signalling in peripheral sensory neurons. Mol. Pain 2020, 16, 1744806920911536. [Google Scholar] [CrossRef]
- Bentley, N.; Awad, A.J.; Patil, P.G. Physiology and pathophysiology of chronic pain. In Neuromodulation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 565–573. [Google Scholar] [CrossRef]
- Greenamyre, J.T.; Maragos, W.F.; Albin, R.L.; Penney, J.B.; Young, A.B. Glutamate transmission and toxicity in Alzheimer’s disease. Prog. Neuro Psychopharmacol. Biol. Psychiatry 1988, 12, 421–430. [Google Scholar] [CrossRef]
- Estrada-Sánchez, A.M.; Montiel, T.; Segovia, J.; Massieu, L. Glutamate toxicity in the striatum of the R6/2 Huntington’s disease transgenic mice is age-dependent and correlates with decreased levels of glutamate transporters. Neurobiol. Dis. 2009, 34, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.; Sobrino, T.; Ramos-Cabrer, P.; Argibay, B.; Agulla, J.; Pérez-Mato, M.; Rodríguez-González, R.; Brea, D.; Castillo, J. Neuroprotection by glutamate oxaloacetate transaminase in ischemic stroke: An experimental study. J. Cereb. Blood Flow Metab. 2011, 31, 1378–1386. [Google Scholar] [CrossRef]
- Takano, T.; Lin, J.H.-C.; Arcuino, G.; Gao, Q.; Yang, J.; Nedergaard, M. Glutamate release promotes growth of malignant gliomas. Nat. Med. 2001, 7, 1010–1015. [Google Scholar] [CrossRef]
- Tucci, S.; Pinto, C.; Goyo, J.; Rada, P.; Hernández, L. Measurement of glutamine and glutamate by capillary electrophoresis and laser induced fluorescence detection in cerebrospinal fluid of meningitis sick children. Clin. Biochem. 1998, 31, 143–150. [Google Scholar] [CrossRef]
- Bonizzoni, M.; Fabbrizzi, L.; Piovani, G.; Taglietti, A. Fluorescent detection of glutamate with a dicopper (II) polyamine cage. Tetrahedron 2004, 60, 11159–11162. [Google Scholar] [CrossRef]
- Xin, L.; Jie, L.; Liu, C.-W.; Zhao, S.-L. Determination of D-Aspartic acid and D-Glutamic acid in midbrain of Parkinson’s disease mouse by reversed phase high performance liquid chromatography. Chin. J. Anal. Chem. 2007, 35, 1151–1154. [Google Scholar] [CrossRef]
- Budczies, J.; Pfitzner, B.M.; Györffy, B.; Winzer, K.J.; Radke, C.; Dietel, M.; Fiehn, O.; Denkert, C. Glutamate enrichment as new diagnostic opportunity in breast cancer. Int. J. Cancer 2015, 136, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Bucher, E.S.; Wightman, R.M. Electrochemical analysis of neurotransmitters. Annu. Rev. Anal. Chem. 2015, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Shadlaghani, A.; Farzaneh, M.; Kinser, D.; Reid, R.C. Direct electrochemical detection of glutamate, acetylcholine, choline, and adenosine using non-enzymatic electrodes. Sensors 2019, 19, 447. [Google Scholar] [CrossRef] [PubMed]
- Isoaho, N.; Peltola, E.; Sainio, S.; Koskinen, J.; Laurila, T. Pt-grown carbon nanofibers for enzymatic glutamate biosensors and assessment of their biocompatibility. RSC Adv. 2018, 8, 35802–35812. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Shahrokhian, S.; Iraji zad, A.; Mohajerzadeh, S.; Vosoughi, M.; Darbari, S.; Koohsorkhi, J.; Mehran, M. Fabrication of sensitive glutamate biosensor based on vertically aligned CNT nanoelectrode array and investigating the effect of CNTs density on the electrode performance. Anal. Chem. 2012, 84, 5932–5938. [Google Scholar] [CrossRef]
- Schultz, J.; Uddin, Z.; Singh, G.; Howlader, M.M. Glutamate sensing in biofluids: Recent advances and research challenges of electrochemical sensors. Analyst 2020, 145, 321–347. [Google Scholar] [CrossRef]
- Kiyatkin, E.A.; Wakabayashi, K.T.; Lenoir, M. Physiological fluctuations in brain temperature as a factor affecting electrochemical evaluations of extracellular glutamate and glucose in behavioral experiments. ACS Chem. Neurosci. 2013, 4, 652–665. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V. Enzyme biosensors for biomedical applications: Strategies for safeguarding analytical performances in biological fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef]
- Jamal, M.; Hasan, M.; Mathewson, A.; Razeeb, K.M. Disposable sensor based on enzyme-free Ni nanowire array electrode to detect glutamate. Biosens. Bioelectron. 2013, 40, 213–218. [Google Scholar] [CrossRef]
- Jamal, M.; Chakrabarty, S.; Shao, H.; McNulty, D.; Yousuf, M.A.; Furukawa, H.; Khosla, A.; Razeeb, K.M. A non enzymatic glutamate sensor based on nickel oxide nanoparticle. Microsyst. Technol. 2018, 24, 4217–4223. [Google Scholar] [CrossRef]
- Hussain, M.M.; Rahman, M.M.; Asiri, A.M.; Awual, M.R. Non-enzymatic simultaneous detection of L-glutamic acid and uric acid using mesoporous Co 3 O 4 nanosheets. RSC Adv. 2016, 6, 80511–80521. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hussain, M.M.; Asiri, A.M.; Alamry, K.; Hasnat, M. An enzyme free detection of L-Glutamic acid using deposited CuO. GdO nanospikes on a flat glassy carbon electrode. Surf. Interfaces 2020, 20, 100617. [Google Scholar] [CrossRef]
- Zeynaloo, E.; Yang, Y.-P.; Dikici, E.; Landgraf, R.; Bachas, L.G.; Daunert, S. Design of a mediator-free, non-enzymatic electrochemical biosensor for glutamate detection. Nanomed. Nanotechnol. Biol. Med. 2021, 31, 102305. [Google Scholar] [CrossRef] [PubMed]
- Maduraiveeran, G.; Jin, W. Nanomaterials based electrochemical sensor and biosensor platforms for environmental applications. Trends Environ. Anal. Chem. 2017, 13, 10–23. [Google Scholar] [CrossRef]
- Zheng, D.; Vashist, S.K.; Dykas, M.M.; Saha, S.; Al-Rubeaan, K.; Lam, E.; Luong, J.H.; Sheu, F.-S. Graphene versus multi-walled carbon nanotubes for electrochemical glucose biosensing. Materials 2013, 6, 1011–1027. [Google Scholar] [CrossRef]
- Buch, M.; Rishpon, J. An electrochemical immunosensor for C-reactive protein based on multi-walled carbon nanotube-modified electrodes. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2008, 20, 2592–2594. [Google Scholar] [CrossRef]
- Hughes, G.; Pemberton, R.; Fielden, P.; Hart, J.P. Development of a novel reagentless, screen-printed amperometric biosensor based on glutamate dehydrogenase and NAD+, integrated with multi-walled carbon nanotubes for the determination of glutamate in food and clinical applications. Sens. Actuators B Chem. 2015, 216, 614–621. [Google Scholar] [CrossRef]
- Bizid, S.; Blili, S.; Mlika, R.; Said, A.H.; Korri-Youssoufi, H. Direct E-DNA sensor of Mycobacterium tuberculosis mutant strain based on new nanocomposite transducer (Fc-ac-OMPA/MWCNTs). Talanta 2018, 184, 475–483. [Google Scholar] [CrossRef]
- Cai, C.; Chen, J. Direct electron transfer of glucose oxidase promoted by carbon nanotubes. Anal. Biochem. 2004, 332, 75–83. [Google Scholar] [CrossRef]
- Oliveira, T.M.; Morais, S. New generation of electrochemical sensors based on multi-walled carbon nanotubes. Appl. Sci. 2018, 8, 1925. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Functionalization of tungsten oxide into MWCNT and its application for sunlight-induced degradation of rhodamine B. J. Colloid Interface Sci. 2011, 362, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Choubey, A.; Zhong, Z.; Pecht, M.G. Copper Wire Bonding. In Copper Wire Bonding; Springer: New York, NY, USA, 2014; pp. 1–9. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.; Li, T.; Guo, Q.; Yang, J. Fabrication of flexible copper-based electronics with high-resolution and high-conductivity on paper via inkjet printing. J. Mater. Chem. C 2014, 2, 286–294. [Google Scholar] [CrossRef]
- Kang, W.; Pei, X.; Rusinek, C.A.; Bange, A.; Haynes, E.N.; Heineman, W.R.; Papautsky, I. Determination of lead with a copper-based electrochemical sensor. Anal. Chem. 2017, 89, 3345–3352. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.R. Copper and Copper Alloys; ASM International: Almere, The Netherlands, 2001. [Google Scholar]
- Magdassi, S.; Grouchko, M.; Kamyshny, A. Copper nanoparticles for printed electronics: Routes towards achieving oxidation stability. Materials 2010, 3, 4626–4638. [Google Scholar] [CrossRef]
- Dharmadasa, R.; Jha, M.; Amos, D.A.; Druffel, T. Room temperature synthesis of a copper ink for the intense pulsed light sintering of conductive copper films. ACS Appl. Mater. Interfaces 2013, 5, 13227–13234. [Google Scholar] [CrossRef]
- Iijima, J.; Lim, J.-W.; Hong, S.-H.; Suzuki, S.; Mimura, K.; Isshiki, M. Native oxidation of ultra high purity Cu bulk and thin films. Appl. Surf. Sci. 2006, 253, 2825–2829. [Google Scholar] [CrossRef]
- Alam, M.M.; Howlader, M.M. Nonenzymatic electrochemical sensors via Cu native oxides (CuNOx) for sweat glucose monitoring. Sens. Bio Sens. Res. 2021, 34, 100453. [Google Scholar] [CrossRef]
- Zhuang, Z.; Su, X.; Yuan, H.; Sun, Q.; Xiao, D.; Choi, M.M. An improved sensitivity non-enzymatic glucose sensor based on a CuO nanowire modified Cu electrode. Analyst 2008, 133, 126–132. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Liu, X.; Wu, J.; Li, M.; Gu, J.; Liu, H.; Fang, B. Different CuO nanostructures: Synthesis, characterization, and applications for glucose sensors. J. Phys. Chem. C 2008, 112, 16845–16849. [Google Scholar] [CrossRef]
- George, A.; Raj, D.M.A.; Raj, A.D.; Irudayaraj, A.A.; Arumugam, J.; Prabu, H.J.; Sundaram, S.J.; Al-Dhabi, N.A.; Arasu, M.V.; Maaza, M. Temperature effect on CuO nanoparticles: Antimicrobial activity towards bacterial strains. Surf. Interfaces 2020, 21, 100761. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-based nanoparticles: Synthesis and applications in catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Zampardi, G.; Thöming, J.; Naatz, H.; Amin, H.M.; Pokhrel, S.; Mädler, L.; Compton, R.G. Electrochemical behavior of single CuO nanoparticles: Implications for the assessment of their environmental fate. Small 2018, 14, 1801765. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Maham, M.; Sajadi, S.M. Green synthesis of CuO nanoparticles by aqueous extract of Gundelia tournefortii and evaluation of their catalytic activity for the synthesis of N-monosubstituted ureas and reduction of 4-nitrophenol. J. Colloid Interface Sci. 2015, 455, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Vidhyadharan, B.; Misnon, I.I.; Abd Aziz, R.; Padmasree, K.; Yusoff, M.M.; Jose, R. Superior supercapacitive performance in electrospun copper oxide nanowire electrodes. J. Mater. Chem. A 2014, 2, 6578–6588. [Google Scholar] [CrossRef]
- Abd El Haleem, S.; Ateya, B.G. Cyclic voltammetry of copper in sodium hydroxide solutions. J. Electroanal. Chem. Interfacial Electrochem. 1981, 117, 309–319. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Balasubramanian, K. Cyclic voltammetric behavior of copper powder immobilized on paraffin impregnated graphite electrode in dilute alkali solution. Int. J. Electrochem. Sci. 2008, 3, 1277–1287. Available online: http://www.electrochemsci.org/list08.htm (accessed on 2 February 2023).
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980; Volume 2. [Google Scholar]
- Laviron, E. Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J. Electroanal. Chem. Interfacial Electrochem. 1974, 52, 355–393. [Google Scholar] [CrossRef]
- Neuberger, A. Dissociation constants and structures of glutamic acid and its esters. Biochem. J. 1936, 30, 2085. [Google Scholar] [CrossRef]
- Brown, W.H.; Iverson, B.L.; Anslyn, E.; Foote, C.S. Organic Chemistry; Cengage Learning: Mason, OH, USA, 2013. [Google Scholar]
- Brownson, D.A.; Banks, C.E. Interpreting electrochemistry. In The Handbook of Graphene Electrochemistry; Springer: Cham, Switzerland, 2014; pp. 23–77. [Google Scholar] [CrossRef]
- Manalastas, W., Jr.; Kumar, S.; Verma, V.; Zhang, L.; Yuan, D.; Srinivasan, M. Water in Rechargeable Multivalent-Ion Batteries: An Electrochemical Pandora’s Box. ChemSusChem 2019, 12, 379–396. [Google Scholar] [CrossRef]
- Kear, G.; Barker, B.; Walsh, F. Electrochemical corrosion of unalloyed copper in chloride media—A critical review. Corros. Sci. 2004, 46, 109–135. [Google Scholar] [CrossRef]
- Lu, Y.; Partridge, C.; Meyyappan, M.; Li, J. A carbon nanotube sensor array for sensitive gas discrimination using principal component analysis. J. Electroanal. Chem. 2006, 593, 105–110. [Google Scholar] [CrossRef]














| Electrode | Detection Method | LOD (µM) | LR (µM) | Sensitivity (μA·mM−1·cm−2) | Electrolyte | Refs. |
|---|---|---|---|---|---|---|
| Pt/NiNAE | Amperometric | 83 | 500–8000 | 96 | 1 M NaOH | [20] |
| NiNAE | Amperometric | 135 | 500–8000 | 65 | 1 M NaOH | [20] |
| NiO/GCE | Amperometric | 272 | 1000–8000 | 11 | 0.1 M NaOH | [21] |
| Co3O4-NS/GCE | I-V | 10 × 10−6 | 10 × 10−4–105 | 9.5 × 10−2 | 0.1 M PBS, pH 7 | [22] |
| CuO.GdO NSs/Nafion/GCE | I-V | 166 × 10−6 | 166 × 10−6–100 × 103 | 0.567 | pH 7 buffer | [23] |
| GluBP/AuNP/SPCE | CV | 0.15 | 0.1–0.8 | 0.05 M PB, pH 7.4 | [24] | |
| CuO-MWCNTs/SPCE | LSV | 17.5 | 20–200 | 8500 | 0.1 M KCl | This Work |
| Added (μM) | Found (μM) a | Recovery (%) |
|---|---|---|
| 40 μM | 36.8 | 92 |
| 60 μM | 67.38 | 112 |
| 100 μM | 96.29 | 96 |
| 140 μM | 142.44 | 101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.Y.; Knight, D.; Howlader, M.M.R. Nonenzymatic Electrochemical Glutamate Sensor Using Copper Oxide Nanomaterials and Multiwall Carbon Nanotubes. Biosensors 2023, 13, 237. https://doi.org/10.3390/bios13020237
Ali MY, Knight D, Howlader MMR. Nonenzymatic Electrochemical Glutamate Sensor Using Copper Oxide Nanomaterials and Multiwall Carbon Nanotubes. Biosensors. 2023; 13(2):237. https://doi.org/10.3390/bios13020237
Chicago/Turabian StyleAli, Md Younus, Dorian Knight, and Matiar M. R. Howlader. 2023. "Nonenzymatic Electrochemical Glutamate Sensor Using Copper Oxide Nanomaterials and Multiwall Carbon Nanotubes" Biosensors 13, no. 2: 237. https://doi.org/10.3390/bios13020237
APA StyleAli, M. Y., Knight, D., & Howlader, M. M. R. (2023). Nonenzymatic Electrochemical Glutamate Sensor Using Copper Oxide Nanomaterials and Multiwall Carbon Nanotubes. Biosensors, 13(2), 237. https://doi.org/10.3390/bios13020237