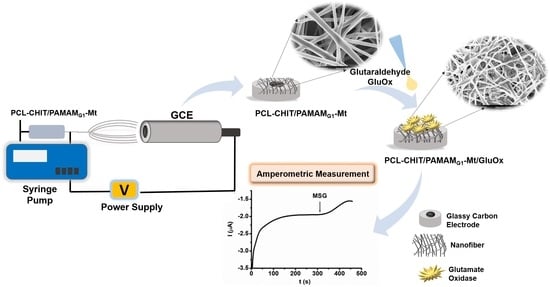
Development of an Enzymatic Biosensor Using Glutamate Oxidase on Organic–Inorganic-Structured, Electrospun Nanofiber-Modified Electrodes for Monosodium Glutamate Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Modification of Mt with PAMAMG1 Dendrimer
2.4. Preparation of the PCL–CHIT/PAMAMG1–Mt/GluOx Biosensors
2.5. Electrochemical Measurements
3. Results
3.1. Characterization of PAMAMG1–Mt
3.2. Formation of PCL–CHIT/PAMAMG1–Mt and GluOx Conjugation on ESNFs
3.3. PCL–CHIT/PAMAMG1–Mt/GluOx for MSG Detection
3.4. PCL–CHIT/PAMAMG1–Mt/GluOx for MSG Detection in Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Fan, Y.; Chen, G.; Liu, Y. Highly Sensitive Glutamate Biosensor Based on Platinum Nanoparticles Decorated MXene-Ti3C2Tx for l-Glutamate Determination in Foodstuffs. LWT 2021, 148, 111748. [Google Scholar] [CrossRef]
- Greenamyre, J.T.; Penney, J.B.; Young, A.B.; D’Amato, C.J.; Hicks, S.P.; Shoulson, I. Alterations in L-Glutamate Binding in Alzheimer’s and Huntington’s Diseases. Science 1985, 227, 1496–1499. [Google Scholar] [CrossRef] [PubMed]
- Pépin, J.; Francelle, L.; Carrillo-de Sauvage, M.-A.; de Longprez, L.; Gipchtein, P.; Cambon, K.; Valette, J.; Brouillet, E.; Flament, J. In Vivo Imaging of Brain Glutamate Defects in a Knock-in Mouse Model of Huntington’s Disease. NeuroImage 2016, 139, 53–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thongsepee, N.; Martviset, P.; Chantree, P.; Sornchuer, P.; Sangpairoj, K.; Prathaphan, P.; Ruangtong, J.; Hiranyachattada, S. Daily Con-sumption of Monosodium Glutamate Pronounced Hypertension and Altered Renal Excretory Function in Normotensive and Hypertensive Rats. Heliyon 2022, 8, e10972. [Google Scholar] [CrossRef]
- Baad-Hansen, L.; Cairns, B.; Ernberg, M.; Svensson, P. Effect of Systemic Monosodium Glutamate (MSG) on Headache and Pericranial Muscle Sensitivity. Cephalalgia 2010, 30, 68–76. [Google Scholar] [CrossRef]
- Zanfirescu, A.; Ungurianu, A.; Tsatsakis, A.M.; Nițulescu, G.M.; Kouretas, D.; Veskoukis, A.; Tsoukalas, D.; Engin, A.B.; Aschner, M.; Margină, D. A Review of the Alleged Health Hazards of Monosodium Glutamate. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1111–1134. [Google Scholar] [CrossRef] [Green Version]
- Kurkinen, M. Astrocyte Glutamate Transporter EAAT2 in Alzheimer Dementia. In Glutamate and Neuropsychiatric Disorders: Current and Emerging Treatments; Pavlovic, Z.M., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 229–259. ISBN 978-3-030-87480-3. [Google Scholar]
- Scoggin, J.L.; Tan, C.; Nguyen, N.H.; Kansakar, U.; Madadi, M.; Siddiqui, S.; Arumugam, P.U.; DeCoster, M.A.; Murray, T.A. An Enzyme-Based Electrochemical Biosensor Probe with Sensitivity to Detect Astrocytic versus Glioma Uptake of Glutamate in Real Time in Vitro. Biosens. Bioelectron. 2019, 126, 751–757. [Google Scholar] [CrossRef]
- Chung, Y.; Yu, D.; Kwak, H.S.; Park, S.S.; Shin, E.C.; Lee, Y. Effect of Monosodium Glutamate on Salt and Sugar Content Reduction in Cooked Foods for the Sensory Characteristics andConsumer Acceptability. Foods 2022, 11, 2512. [Google Scholar] [CrossRef]
- Abdallah, C.G.; Jiang, L.; De Feyter, H.M.; Fasula, M.; Krystal, J.H.; Rothman, D.L.; Mason, G.F.; Sanacora, G. Glutamate Metabolism in Major Depressive Disorder. AJP 2014, 171, 1320–1327. [Google Scholar] [CrossRef] [Green Version]
- Okon, S.L.; Ronkainen, N.J.; Okon, S.L.; Ronkainen, N.J. Enzyme-Based Electrochemical Glutamate Biosensors; IntechOpen: London, UK, 2017; ISBN 978-953-51-3194-6. [Google Scholar]
- Nanda, P.K.; Bhattacharya, D.; Das, J.K.; Bandyopadhyay, S.; Ekhlas, D.; Lorenzo, J.M.; Dandapat, P.; Alessandroni, L.; Das, A.K.; Ga-gaoua, M. Emerging Role of Biosensors and Chemical Indicators to Monitor the Quality and Safety of Meat and Meat Products. Chemosensors 2022, 10, 322. [Google Scholar] [CrossRef]
- Devi, R.; Gogoi, S.; Barua, S.; Sankar Dutta, H.; Bordoloi, M.; Khan, R. Electrochemical Detection of Monosodium Glutamate in Foodstuffs Based on Au@MoS2/Chitosan Modified Glassy Carbon Electrode. Food Chem. 2019, 276, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Devi, R.; Jaiswal, J.; Dutta, H.S.; Khan, R.; Dhayal, M. A Highly Sensitive Immunosensor Based on In Situ Reduced Gold-Chitosan Nanocomposite for Detection of Monosodium L-Glutamate. J. Biosyst. Eng. 2022, 47, 28–38. [Google Scholar] [CrossRef]
- Li, P.; Jia, J.; Geng, Z.; Pang, S.; Wang, R.; Bilal, M.; Bian, H.; Cui, J.; Jia, S. A Dual Enzyme-Phosphate Hybrid Nanoflower for Glutamate Detection. In Particuology; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Santos, F.D.S.; Silva, L.V.d.; Campos, P.V.S.; Strunkis, C.d.M.; Ribeiro, C.M.G.; Salles, M.O. Review—Recent Advances of Electro-chemical Techniques in Food, Energy, Environment, and Forensic Applications. ECS Sens. Plus 2022, 1, 013603. [Google Scholar] [CrossRef]
- Demirkol, D.O.; Yildiz, H.B.; Sayın, S.; Yilmaz, M. Enzyme Immobilization in Biosensor Constructions: Self-Assembled Monolayers of Calixarenes Containing Thiols. RSC Adv. 2014, 4, 19900–19907. [Google Scholar] [CrossRef]
- Oner, A.; Tufek, E.; Yezer, I.; Birol, A.; Demir, M.; Er, S.; Demirkol, D.O. High Generation Dendrimer Decorated Poly-Ɛ-Caprolactone/Polyacrylic Acid Electrospun Nanofibers for the Design of a Bioelectrochemical Sensing Surface. React. Funct. Polym. 2021, 161, 104853. [Google Scholar] [CrossRef]
- Serafín, V.; Valverde, A.; Martínez-García, G.; Martínez-Periñán, E.; Comba, F.; Garranzo-Asensio, M.; Barderas, R.; Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Graphene Quantum Dots-Functionalized Multi-Walled Carbon Nanotubes as Nanocarriers in Electrochemi-cal Immunosensing. Determination of IL-13 Receptor A2 in Colorectal Cells and Tumor Tissues with Different Metastatic Potential. Sens. Actuators Chem. 2019, 284, 711–722. [Google Scholar] [CrossRef]
- Kırgöz, Ü.A.; Odacı, D.; Timur, S.; Merkoçi, A.; Pazarlıoğlu, N.; Telefoncu, A.; Alegret, S. Graphite Epoxy Composite Electrodes Modified with Bacterial Cells. Bioelectrochemistry 2006, 69, 128–131. [Google Scholar] [CrossRef]
- Çakar, İ.; Özdokur, K.V.; Demir, B.; Yavuz, E.; Demirkol, D.O.; Koçak, S.; Timur, S.; Ertaş, F.N. Molybdenum Oxide/Platinum Modified Glassy Carbon Electrode: A Novel Electrocatalytic Platform for the Monitoring of Electrochemical Reduction of Oxygen and Its Biosensing Applications. Sens. Actuators Chem. 2013, 185, 331–336. [Google Scholar] [CrossRef]
- Bongartz, R.; Ag, D.; Seleci, M.; Walter, J.-G.; Yalcinkaya, E.E.; Demirkol, D.O.; Stahl, F.; Timur, S.; Scheper, T. Folic Acid-Modified Clay: Targeted Surface Design for Cell Culture Applications. J. Mater. Chem. 2012, 1, 522–528. [Google Scholar] [CrossRef]
- Sonmez, B.; Sayin, S.; Yalcinkaya, E.E.; Seleci, D.A.; Yildiz, H.B.; Demirkol, D.O.; Timur, S. Calixarene Modified Montmorillonite: A Novel Design for Biosensing Applications. RSC Adv. 2014, 4, 62895–62902. [Google Scholar] [CrossRef]
- Yilmaz, Y.Y.; Yalcinkaya, E.E.; Demirkol, D.O.; Timur, S. 4-Aminothiophenol-Intercalated Montmorillonite: Organic-Inorganic Hybrid Material as an Immobilization Support for Biosensors. Sens. Actuators Chem. 2020, 307, 127665. [Google Scholar] [CrossRef]
- Demir, B.; Seleci, M.; Ag, D.; Cevik, S.; Yalcinkaya, E.E.; Demirkol, D.O.; Anik, U.; Timur, S. Amine Intercalated Clay Surfaces for Mi-crobial Cell Immobilization and Biosensing Applications. RSC Adv. 2013, 3, 7513–7519. [Google Scholar] [CrossRef]
- Songurtekin, D.; Yalcinkaya, E.E.; Ag, D.; Seleci, M.; Demirkol, D.O.; Timur, S. Histidine modified montmorillonite: Laccase immobilization and application to flow injection analysis of phenols. Appl. Clay Sci. 2013, 86, 64–69. [Google Scholar] [CrossRef]
- Seleci, M.; Ag, D.; Yalcinkaya, E.E.; Demirkol, D.O.; Guler, C.; Timur, S. Amine-intercalated montmorillonite matrices for enzyme immobilization and biosensing applications. RSC Adv. 2012, 2, 2112–2118. [Google Scholar] [CrossRef]
- Unal, B.; Yalcinkaya, E.E.; Gumustas, S.; Sonmez, B.; Ozkan, M.; Balcan, M.; Demirkol, D.O.; Timur, S. Polyglycolide–montmorillonite as a novel nanocomposite platform for biosensing applications. New J. Chem. 2017, 41, 9371–9379. [Google Scholar] [CrossRef]
- Unal, B.; Yalcinkaya, E.E.; Demirkol, D.O.; Timur, S. An Electrospun Nanofiber Matrix Based on Organo-Clay for Biosensors: PVA/PAMAM-Montmorillonite. Appl. Surf. Sci. 2018, 444, 542–551. [Google Scholar] [CrossRef]
- Kirbay, F.O.; Yalcinkaya, E.E.; Atik, G.; Evren, G.; Unal, B.; Demirkol, D.O.; Timur, S. Biofunctionalization of PAMAM-Montmorillonite Decorated Poly (Ɛ-Caprolactone)-Chitosan Electrospun Nanofibers for Cell Adhesion and Electrochemical Cytosensing. Biosens. Bioelectron. 2018, 109, 286–294. [Google Scholar] [CrossRef]
- Damar, K.; Odaci Demirkol, D. Modified Gold Surfaces by Poly(Amidoamine) Dendrimers and Fructose Dehydrogenase for Mediated Fructose Sensing. Talanta 2011, 87, 67–73. [Google Scholar] [CrossRef]
- Hatamluyi, B.; Es’haghi, Z. Quantitative Biodetection of Anticancer Drug Rituxan with DNA Biosensor Modified PAMAM Den-drimer/Reduced Graphene Oxide Nanocomposite. Electroanalysis 2018, 30, 1659–1668. [Google Scholar] [CrossRef]
- Malvano, F.; Pilloton, R.; Rubino, A.; Albanese, D. Rapid Detection of Deoxynivalenol in Dry Pasta Using a Label-Free Immunosensor. Biosensors 2022, 12, 240. [Google Scholar] [CrossRef]
- Wang, X.; Shi, W.; Wang, Y.; Cheng, D.; Liu, J.; Xu, S.; Liu, W.; Dong, B.; Sun, J. Intrinsic Blue Fluorescence of 2.0G PAMAM-DCM Polymer Dots and Its Applications for Fe3+ Sensing. Sensors 2022, 22, 1075. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.O.; Akanji, S.P.; Orimolade, B.O.; Olorundare, F.O.G.; Azizi, S.; Mamba, B.; Maaza, M. Using Nanomaterials as Excellent Immobilisation Layer for Biosensor Design. Biosensors 2023, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, X.; Liu, P.; Yu, D.-G.; Ge, R. Electrospun Nanofiber-Based Glucose Sensors for Glucose Detection. Front. Chem. 2022, 10, 944428. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Nanofiber-Based Chemical Sensors. In Applications of Polymer Nanofibers; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 100–134. ISBN 978-1-119-26771-3. [Google Scholar]
- Kim, D.-H.; Bae, J.; Lee, J.; Ahn, J.; Hwang, W.-T.; Ko, J.; Kim, I.-D. Porous Nanofiber Membrane: Rational Platform for Highly Sensitive Thermochromic Sensor. Adv. Funct. Mater. 2022, 32, 2200463. [Google Scholar] [CrossRef]
- Chokkiah, B.; Eswaran, M.; Wabaidur, S.M.; Alothman, Z.A.; Lee, S.C.; Dhanusuraman, R. An Efficient Amperometric Sensor for Chloride Ion Detection through Electroactive E-Spun PVA-PANi-g-C3N4 Nanofiber. J. Mater. Sci. Mater. Electron. 2022, 33, 9425–9437. [Google Scholar] [CrossRef]
- Yezer, I.; Demirkol, D.O. Cellulose Acetate–Chitosan Based Electrospun Nanofibers for Bio-Functionalized Surface Design in Biosensing. Cellulose 2020, 27, 10183–10197. [Google Scholar] [CrossRef]
- Du, J.; Tan, E.; Kim, H.J.; Zhang, A.; Bhattacharya, R.; Yarema, K.J. Comparative Evaluation of Chitosan, Cellulose Acetate, and Polyether-sulfone Nanofiber Scaffolds for Neural Differentiation. Carbohydr. Polym. 2014, 99, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Shabanloo, R.; Akbari, S.; Mirsalehi, M. Hybrid electrospun scaffolds based on polylactic acid/PAMAM dendrimer/gemini surfactant for enhancement of synergistic antibacterial ability for biomedical application. Biomed. Mater. 2022, 17, 045009. [Google Scholar] [CrossRef]
- Priya; Gogate, P.R. Ultrasound-Assisted Intensification of β-Glucosidase Enzyme Activity in Free and Immobilized Forms. Ind. Eng. Chem. Res. 2022, 61, 2023–2036. [Google Scholar] [CrossRef]
- Tangaraj, V.; Janot, J.-M.; Jaber, M.; Bechelany, M.; Balme, S. Adsorption and Photophysical Properties of Fluorescent Dyes over Montmo-rillonite and Saponite Modified by Surfactant. Chemosphere 2017, 184, 1355–1361. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical Properties and Antioxidant Activity of Chitosan from the Blowfly Chrysomya megacephala Larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.S.S.P.; Costa, L.S.; Cordeiro, S.L.; Almeida-Lima, J.; Dantas-Santos, N.; Magalhães, K.D.; Sabry, D.A.; Albuquerque, I.R.L.; Pe-reira, M.R.; Leite, E.L.; et al. Evaluating the Possible Anticoagulant and Antioxidant Effects of Sulfated Polysaccharides from the Tropical Green Alga Caulerpa Cupressoides Var. Flabellata. J. Appl. Phycol. 2012, 24, 1159–1167. [Google Scholar] [CrossRef]
- Jeon, H.J.; Kim, J.S.; Kim, T.G.; Kim, J.H.; Yu, W.-R.; Youk, J.H. Preparation of Poly(ɛ-Caprolactone)-Based Polyurethane Nanofibers Containing Silver Nanoparticles. Appl. Surf. Sci. 2008, 254, 5886–5890. [Google Scholar] [CrossRef]
- Quoc, P.L.; Solovieva, A.Y.; Uspenskaya, M.V.; Olekhnovich, R.O.; Sitnikova, V.E.; Strelnikova, I.E.; Kunakova, A.M. High-Porosity Polymer Composite for Removing Oil Spills in Cold Regions. ACS Omega 2021, 6, 20512–20521. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, B.; Wang, Y.; Qi, G.; Tian, F.; Yang, J.; Wang, S. Co-continuous structural electrolytes based on ionic liquid, epoxy resin and organoclay: Effects of organoclay content. Mater. Des. 2016, 104, 126–133. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, G.; Wang, J.; Xie, C.; Liang, W.; Chen, K.; Wen, Y.; Li, X. Organic Solvent-Free Preparation of Chitosan Nanofibers with High Specific Surface Charge and Their Application in Biomaterials. ACS Appl. Mater. Interfaces 2021, 13, 12347–12358. [Google Scholar] [CrossRef]
- Yotsuji, K.; Tachi, Y.; Sakuma, H.; Kawamura, K. Effect of interlayer cations on montmorillonite swelling: Comparison between molecular dynamic simulations and experiments. Appl. Clay Sci. 2021, 204, 106034. [Google Scholar] [CrossRef]
- Ali, M.Y.; Knight, D.; Howlader, M.M.R. Nonenzymatic Electrochemical Glutamate Sensor Using Copper Oxide Nanomaterials and Multi-wall Carbon Nanotubes. Biosensors 2023, 13, 237. [Google Scholar] [CrossRef]
- Martínez-Periñán, E.; Domínguez-Saldaña, A.; Villa-Manso, A.M.; Gutiérrez-Sánchez, C.; Revenga-Parra, M.; Mateo-Martí, E.; Pariente, F.; Lorenzo, E. Azure A Embedded in Carbon Dots as NADH Electrocatalyst: Development of a Glutamate Electrochemical Biosensor. Sens. Actuators Chem. 2023, 374, 132761. [Google Scholar] [CrossRef]
- Yang, L.; Bai, R.; Xie, B.; Zhuang, N.; Lv, Z.; Chen, M.; Dong, W.; Zhou, J.; Jiang, M. A Biosensor Based on Oriented Immobilization of an Engineered L-Glutamate Oxidase on a Screen-Printed Microchip for Detection of l-Glutamate in Fermentation Processes. Food Chem. 2023, 405, 134792. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, L.; Ma, Y.; Ye, J. Construction of Minitype Glutamate Sensor for in Vivo Monitoring of L-Glutamate in Plant. Microchem. J. 2023, 188, 108505. [Google Scholar] [CrossRef]
- Mentana, A.; Nardiello, D.; Palermo, C.; Centonze, D. Accurate Glutamate Monitoring in Foodstuffs by a Sensitive and Interference-Free Glutamate Oxidase Based Disposable Amperometric Biosensor. Anal. Chim. Acta 2020, 1115, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Duan, J.; Cai, Y.; Liu, D.; Li, X.; Dong, Y.; Hu, F. A Modified Nanocomposite Biosensor for Quantitative L-Glutamate Detection in Beef. Meat Sci. 2020, 168, 108185. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Dutta, H.S.; Dhayal, M. Langmuir-Blodgett Monolayer of Electrochemically Synthesized PANI-TiO2 Nanocomposites for MSG Biosensor. Appl. Surf. Sci. Adv. 2022, 10, 100264. [Google Scholar] [CrossRef]
- Shigemura, N.; Shirosaki, S.; Sanematsu, K.; Yoshida, R.; Ninomiya, Y. Genetic and Molecular Basis of Individual Differences in Human Umami Taste Perception. PLoS ONE 2009, 4, e6717. [Google Scholar] [CrossRef]








| Material | Detection Mode | Linear Range | LOD | Samples | Ref. |
|---|---|---|---|---|---|
| CuO with MWCNTs | CV | 20–200 µM | 17.5 µM | Whole blood and urine | [52] |
| GLDH/Chit-AA-CDs/SPCE | CV | 11–125 µM | 3.3 µM | Blood serum and barbecue flavored corn snack samles | [53] |
| ChBD-GluOX/PB/SPC | CV | 25 µmol/L to 300 µmol/L | 9.0 µmol/L | Fermentation broth samples | [54] |
| GluOx/PMPD/Pt modified GRE | CV | 2.0–550 μM | 0.536 μM | Cucumber juice and fruit | [55] |
| PtNP decorated MXene-Ti3C2Tx | AMP | 10–110 μmol/L | 0.45 μmol/L | Vegetable soup, soy sauce, stock cube, and mushroom seasoning | [1] |
| Au@MoS2/CS | CV, DPV and EIS | 0.05–200 µM | 0.03 μM | Food | [13] |
| PPy/GluOx | AMP | 5.0 µM–1.0 mM | 1.8 μM | Stock cubes, ketchup and Parmigiano Reggiano chees | [56] |
| AuNPs/GO/CS | CV and DPV | 0.2–1.4 mM | 0.023 mM | Beef | [57] |
| CS-AuNPs | CV, DPV and EIS | 100 pM to 1 μM | - | Freshly prepared tomato sauce | [14] |
| PANI-TiO2 | CV and DPV | 1 nM to 500 µM and 1 µM to 250 µM | 37 mA/nM | Tomato sauce | [58] |
| PCL:CHIT/Mt | AMP | 25 µM to 0.25 mM | 7.019 µM | - | This study |
| PCL:CHIT/PAMAMG1-Mt | AMP | 2.5 µM to 0.175 mM | 1.045 µM | Tomato soup | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atilgan, H.; Unal, B.; Yalcinkaya, E.E.; Evren, G.; Atik, G.; Ozturk Kirbay, F.; Kilic, N.M.; Odaci, D. Development of an Enzymatic Biosensor Using Glutamate Oxidase on Organic–Inorganic-Structured, Electrospun Nanofiber-Modified Electrodes for Monosodium Glutamate Detection. Biosensors 2023, 13, 430. https://doi.org/10.3390/bios13040430
Atilgan H, Unal B, Yalcinkaya EE, Evren G, Atik G, Ozturk Kirbay F, Kilic NM, Odaci D. Development of an Enzymatic Biosensor Using Glutamate Oxidase on Organic–Inorganic-Structured, Electrospun Nanofiber-Modified Electrodes for Monosodium Glutamate Detection. Biosensors. 2023; 13(4):430. https://doi.org/10.3390/bios13040430
Chicago/Turabian StyleAtilgan, Hamdiye, Betul Unal, Esra Evrim Yalcinkaya, Gizem Evren, Gozde Atik, Fatma Ozturk Kirbay, Nur Melis Kilic, and Dilek Odaci. 2023. "Development of an Enzymatic Biosensor Using Glutamate Oxidase on Organic–Inorganic-Structured, Electrospun Nanofiber-Modified Electrodes for Monosodium Glutamate Detection" Biosensors 13, no. 4: 430. https://doi.org/10.3390/bios13040430
APA StyleAtilgan, H., Unal, B., Yalcinkaya, E. E., Evren, G., Atik, G., Ozturk Kirbay, F., Kilic, N. M., & Odaci, D. (2023). Development of an Enzymatic Biosensor Using Glutamate Oxidase on Organic–Inorganic-Structured, Electrospun Nanofiber-Modified Electrodes for Monosodium Glutamate Detection. Biosensors, 13(4), 430. https://doi.org/10.3390/bios13040430