Powder Synthesized from Aqueous Solution of Calcium Nitrate and Mixed-Anionic Solution of Orthophosphate and Silicate Anions for Bioceramics Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Powders
2.3. Preparation of Ceramic Samples
2.4. Methods of Analysis
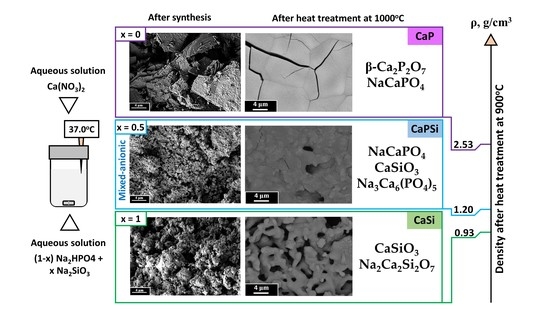
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, M.M. Biomaterials for Bone Tissue Engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Ansari, M. Bone Tissue Regeneration: Biology, Strategies and Interface Studies. Prog. Biomater. 2019, 8, 223–237. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Hasan, A.; Kaarela, O.; Byambaa, B.; Sheikhi, A.; Gaharwar, A.K.; Khademhosseini, A. Advancing Frontiers in Bone Bioprinting. Adv. Healthc. Mater. 2019, 8, 1801048. [Google Scholar] [CrossRef] [PubMed]
- Amiryaghoubi, N.; Fathi, M.; Pesyan, N.N.; Samiei, M.; Barar, J.; Omidi, Y. Bioactive Polymeric Scaffolds for Osteogenic Repair and Bone Regenerative Medicine. Med. Res. Rev. 2020, 40, 1833–1870. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated Degradation of HAP/PLLA Bone Scaffold by PGA Blending Facilitates Bioactivity and Osteoconductivity. Bioact. Mater. 2021, 6, 490–502. [Google Scholar] [CrossRef]
- Yamada, Y.; Inui, T.; Kinoshita, Y.; Shigemitsu, Y.; Honda, M.; Nakano, K.; Matsunari, H.; Nagaya, M.; Nagashima, H.; Aizawa, M. Silicon-Containing Apatite Fiber Scaffolds with Enhanced Mechanical Property Express Osteoinductivity and High Osteoconductivity. J. Asian Ceram. Soc. 2019, 7, 101–108. [Google Scholar] [CrossRef]
- Malhotra, A.; Habibovic, P. Calcium Phosphates and Angiogenesis: Implications and Advances for Bone Regeneration. Trends Biotechnol. 2016, 34, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Bleek, K.; Schell, H.; Schulz, N.; Hoff, P.; Perka, C.; Buttgereit, F.; Volk, H.-D.; Lienau, J.; Duda, G.N. Inflammatory Phase of Bone Healing Initiates the Regenerative Healing Cascade. Cell Tissue Res. 2012, 347, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Mao, M.; He, J.; Li, X.; Zhang, B.; Lei, Q.; Liu, Y.; Li, D. The Emerging Frontiers and Applications of High-Resolution 3D Printing. Micromachines 2017, 8, 113. [Google Scholar] [CrossRef] [Green Version]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed]
- Yunus Basha, R.; Kumar, T.S.S.; Doble, M. Design of Biocomposite Materials for Bone Tissue Regeneration. Mater. Sci. Eng. C 2015, 57, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, C.M.; Nychka, J.A. Bone ‘Spackling’ Paste: Mechanical Properties and In Vitro Response of a Porous Ceramic Composite Bone Tissue Scaffold. J. Mech. Behav. Biomed. Mater. 2020, 112, 103958. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, C.M.; Nychka, J. Fabrication of a Porous and Formable Ceramic Composite Bone Tissue Scaffold at Ambient Temperature. Metall. Mater. Trans. A 2020, 51, 6110–6126. [Google Scholar] [CrossRef]
- Kazakova, G.; Safronova, T.; Golubchikov, D.; Shevtsova, O.; Rau, J.V. Resorbable Mg2+-Containing Phosphates for Bone Tissue Repair. Materials 2021, 14, 4857. [Google Scholar] [CrossRef]
- Zuev, D.M.; Golubchikov, D.O.; Evdokimov, P.V.; Putlyaev, V.I. Synthesis of Amorphous Calcium Phosphate Powders for Production of Bioceramics and Composites by 3D Printing. Russ. J. Inorg. Chem. 2022, 67, 940–951. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials Design for Bone-Tissue Engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Tan, Y.; Wang, J.; Liu, H.; Wang, Y.; Yang, S.; Shi, M.; Zhao, S.; Zhang, Y.; et al. A Difunctional Regeneration Scaffold for Knee Repair Based on Aptamer-Directed Cell Recruitment. Adv. Mater. 2017, 29, 1605235. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding Mechanisms at the Interface of Ceramic Prosthetic Materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Hench, L.L.; Paschall, H.A. Direct Chemical Bond of Bioactive Glass-Ceramic Materials to Bone and Muscle. J. Biomed. Mater. Res. 1973, 7, 25–42. [Google Scholar] [CrossRef]
- Li, H.; Xue, K.; Kong, N.; Liu, K.; Chang, J. Silicate Bioceramics Enhanced Vascularization and Osteogenesis through Stimulating Interactions between Endothelia Cells and Bone Marrow Stromal Cells. Biomaterials 2014, 35, 3803–3818. [Google Scholar] [CrossRef]
- Kaully, T.; Kaufman-Francis, K.; Lesman, A.; Levenberg, S. Vascularization—The Conduit to Viable Engineered Tissues. Tissue Eng. Part B Rev. 2009, 15, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Hillert, M.; Sundman, B.; Wang, X. An Assessment of the CaO-SiO2 System. Metall. Mater. Trans. B 1990, 21, 303–312. [Google Scholar] [CrossRef]
- Fu, S.; Liu, W.; Liu, S.; Zhao, S.; Zhu, Y. 3D Printed Porous β-Ca2SiO4 Scaffolds Derived from Preceramic Resin and Their Physicochemical and Biological Properties. Sci. Technol. Adv. Mater. 2018, 19, 495–506. [Google Scholar] [CrossRef]
- Pan, Y.; Yin, J.; Yao, D.; Zuo, K.; Xia, Y.; Liang, H.; Zeng, Y. Effects of Silica Sol on the Microstructure and Mechanical Properties of CaSiO3 Bioceramics. Mater. Sci. Eng. C. 2016, 64, 336–340. [Google Scholar] [CrossRef]
- De Aza, P.N.; Luklinska, Z.B.; Martinez, A.; Anseau, M.R.; Guitian, F.; De Aza, S. Morphological and Structural Study of Pseudowollastonite Implants in Bone. J. Microsc. 2000, 197, 60–67. [Google Scholar] [CrossRef]
- Yu, Q.; Chang, J.; Wu, C. Silicate Bioceramics: From Soft Tissue Regeneration to Tumor Therapy. J. Mater. Chem. B 2019, 7, 5449–5460. [Google Scholar] [CrossRef]
- Hu, Y.; Xiao, Z.; Wang, H.; Ye, C.; Wu, Y.; Xu, S. Fabrication and Characterization of Porous CaSiO3 Ceramics. Ceram. Int. 2019, 45, 3710–3714. [Google Scholar] [CrossRef]
- Filippov, Y.; Murashko, A.; Evdokimov, P.; Safronova, T.; Putlayev, V. Stereolithography 3D printed calcium pyrophosphate macroporous ceramics for bone grafting. Open Ceram. 2021, 8, 100185. [Google Scholar] [CrossRef]
- Serena, S.; Sainz, M.; Caballero, A. Single-phase silicocarnotite synthesis in the subsystem Ca3(PO4)2–Ca2SiO4. Ceram. Int. 2014, 40, 8245–8252. [Google Scholar] [CrossRef]
- Ros-Tarraga, P.; Mazon, P.; Meseguer-Olmo, L.; De Aza, P. Revising the Subsystem Nurse’s A-Phase-Silicocarnotite within the System Ca3(PO4)2–Ca2SiO4. Materials 2016, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhai, D.; Chang, J.; Wu, C. In Vitro assessment of three-dimensionally plotted nagelschmidtite bioceramic scaffolds with varied macropore morphologies. Acta Biomater. 2014, 10, 463–476. [Google Scholar] [CrossRef]
- Anand, V.; Singh, K.; Kaur, K. Investigation of Mg and Zn doped 45S5 bioactive materials by XRD, FTIR and SEM techniques. AIP Conf. Proc. 2014, 1591, 745. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, X.; Zhang, J.; Fan, X.; Chen, M.; Yang, H. Nucleation, Crystallization and Biological Activity of Na2O-CaO-P2O5-SiO2 Bioactive Glass. J. Non Cryst. Solids 2021, 568, 120929. [Google Scholar] [CrossRef]
- Kahlenberg, V.; Hösch, A. The Crystal Structure of Na2Ca2Si2O7—A Mixed Anion Silicate with Defect Perovskite Characteristics. Z. Krist. Cryst. Mater. 2002, 217, 155–163. [Google Scholar] [CrossRef]
- Leenakul, W.; Pisitpipathsin, N.; Kantha, P.; Tawichai, N.; Tigunta, S.; Eitssayeam, S.; Rujijanagul, G.; Pengpat, K.; Munpakdee, A. Characteristics of 45S5 Bioglass-Ceramics Using Natural Raw Materials. AMR Adv. Mater. Res. 2012, 506, 174–177. [Google Scholar] [CrossRef]
- Kaimonov, M.; Safronova, T.; Shatalova, T.; Filippov, Y.; Tikhomirova, I.; Sergeev, N. Composite Ceramics in the Na2O–CaO–SiO2–P2O5 System Obtained from Pastes Including Hydroxyapatite and an Aqueous Solution of Sodium Silicate. Ceramics 2022, 5, 550–561. [Google Scholar] [CrossRef]
- Safronova, T.V. Inorganic Materials for Regenerative Medicine. Inorg. Mater. 2021, 57, 443–474. [Google Scholar] [CrossRef]
- Demirkiran, H.; Mohandas, A.; Dohi, M.; Fuentes, A.; Nguyen, K.; Aswath, P. Bioactivity and Mineralization of Hydroxyapatite with Bioglass as Sintering Aid and Bioceramics with Na3Ca6(PO4)5 and Ca5(PO4)2SiO4 in a Silicate Matrix. Mater. Sci. Eng. C 2010, 30, 263–272. [Google Scholar] [CrossRef]
- Lin, K.; Zhai, W.; Ni, S.; Chang, J.; Zeng, Y.; Qian, W. Study of the Mechanical Property and In Vitro Biocompatibility of CaSiO3 Ceramics. Ceram. Int. 2005, 31, 323–326. [Google Scholar] [CrossRef]
- Gmeiner, R.; Deisinger, U.; Schönherr, J.; Lechner, B.; Detsch, R.; Boccaccini, A.R.; Stampfl, J. Additive Manufacturing of Bioactive Glasses and Silicate Bioceramics. J. Ceram. Sci. Technol. 2015, 6, 75–86. [Google Scholar] [CrossRef]
- Bergmann, C.; Lindner, M.; Zhang, W.; Koczur, K.; Kirsten, A.; Telle, R.; Fischer, H. 3D Printing of Bone Substitute Implants Using Calcium Phosphate and Bioactive Glasses. J. Eur. Ceram. Soc. 2010, 30, 2563–2567. [Google Scholar] [CrossRef]
- Safronova, T.V.; Knot’ko, A.V.; Shatalova, T.B.; Evdokimov, P.V.; Putlyaev, V.I.; Kostin, M.S. Calcium phosphate ceramic based on powder synthesized from a mixed-anionic solution. Glass Ceram. 2016, 73, 25–31. [Google Scholar] [CrossRef]
- Peranidze, K.; Safronova, T.V.; Filippov, Y.; Kazakova, G.; Shatalova, T.; Rau, J.V. Powders Based on Ca2P2O7-CaCO3-H2O System as Model Objects for the Development of Bioceramics. Ceramics 2022, 5, 423–434. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Filippov, Y.Y.; Knot’Ko, A.V.; Klimashina, E.S.; Peranidze, K.K.; Evdokimov, P.V.; Vladimirova, S.A. Powders Synthesized from Calcium Acetate and Mixed-Anionic Solutions, Containing Orthophosphate and Carbonate Ions, for Obtaining Bioceramic. Glass Ceram. 2018, 75, 118–123. [Google Scholar] [CrossRef]
- Joksa, A.A.; Komarovska, L.; Ubele-Kalnina, D.; Viksna, A.; Gross, K.A. Role of carbonate on the crystallization and processing of amorphous calcium phosphates. Materialia 2023, 27, 101672. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Filippov, Y.; Shatalova, T.B.; Fatin, D.S. Ceramics based on brushite powder synthesized from calcium nitrate and disodium and dipotassium hydrogen phosphates. Inorg. Mater. 2018, 54, 195–207. [Google Scholar] [CrossRef]
- Safronova, T.V. Phase Composition of Ceramic Based on Calcium Hydroxyapatite Powders Containing Byproducts of the Synthesis Reaction. Glass Ceram. 2009, 66, 136–139. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Knot’ko, A.V.; Shatalova, T.B.; Artemov, M.V.; Filippov, Y.Y. Properties of Calcium Phosphate Powder Synthesized from Calcium Chloride and Potassium Pyrophosphate. Inorg. Mater. Appl. Res. 2020, 11, 44–49. [Google Scholar] [CrossRef]
- Gorshkov, V.; Timashev, V.; Saveliev, V. Identification characteristics of compounds containing water. In Methods for Physical and Chemical Analysis of Binders; Gaidzhurov, P., Nekrasov, K., Eds.; Vyshaya Shkola: Moscow, Russia, 1981; pp. 292–294. (In Russian) [Google Scholar]
- Solonenko, A.P.; Blesman, A.I.; Polonyankin, D.A.; Gorbunov, V.A. Synthesis of Calcium Phosphate and Calcium Silicate Composites. Russ. J. Inorg. Chem. 2018, 63, 993–1000. [Google Scholar] [CrossRef]
- Vecstaudza, J.; Gasik, M.; Locs, J. Amorphous Calcium Phosphate Materials: Formation, Structure and Thermal Behaviour. J. Eur. Ceram. Soc. 2019, 39, 1642–1649. [Google Scholar] [CrossRef]
- ICDD. PDF-4+ Database; Kabekkodu, S., Ed.; International Centre for Diffraction Data: Newtown Square, PA, USA, 2010; Available online: https://www.icdd.com/pdf-2/ (accessed on 20 February 2022).
- Li, L.; Hu, H.; Zhu, Y.; Zhu, M.; Liu, Z. 3D-Printed Ternary SiO2CaO P2O5 Bioglass-Ceramic Scaffolds with Tunable Compositions and Properties for Bone Regeneration. Ceram. Int. 2019, 45, 10997–11005. [Google Scholar] [CrossRef]








| No. | Labeling | Concentration × Volume | ||
|---|---|---|---|---|
| Na2SiO3 | Na2HPO4 | Ca(NO3)2 | ||
| 1 | CaP | - | 0.5 M × 0.5 L | 0.5 M × 0.5 L |
| 2 | CaPSi | 0.5 M × 0.25 L | 0.5 M × 0.25 L | 0.5 M × 0.5 L |
| 3 | CaSi | 0.5 M × 0.5 L | - | 0.5 M × 0.5 L |
| No. | Labeling | Weight of Prepared Powders, g | Calculated Weight of By-Product, g | Weight of Collected By-Product, g | Difference in Weight of By-Product, g |
|---|---|---|---|---|---|
| 1 | CaP | 41.97 | 42.44 | 40.01 | 2.44 |
| 2 | CaPSi | 47.89 | 42.44 | 32.89 | 9.55 |
| 3 | CaSi | 62.76 | 42.44 | 22.05 | 20.39 |
| Sample | Heat Treatment Temperature, °C | ||||
|---|---|---|---|---|---|
| 400 | 600 | 800 | 900 | 1000 | |
| CaP | β-Ca2P2O7 NaNO3 | β-Ca2P2O7 β-NaCaPO4 | β-Ca2P2O7 β-NaCaPO4 | β-Ca2P2O7 β-NaCaPO4 | β-Ca2P2O7 β-NaCaPO4 |
| CaPSi | Ca5(PO4)3OH NaNO3 α-CaSiO3 | β-NaCaPO4 Ca5(PO4)3OH α-CaSiO3 | β-NaCaPO4 Ca5(PO4)3OH α-CaSiO3 Na3Ca6(PO4)5 | β-NaCaPO4 α-CaSiO3 Na3Ca6(PO4)5 | β-NaCaPO4 α-CaSiO3 Na3Ca6(PO4)5 |
| CaSi | CSH NaNO3 | CSH NaNO3 Ca5Si6O16(OH)2 Na6Ca3Si6O18 | α-CaSiO3 Na2Ca2Si2O7 | α-CaSiO3 Na2Ca2Si2O7 | α-CaSiO3 Na2Ca2Si2O7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubchikov, D.; Safronova, T.V.; Nemygina, E.; Shatalova, T.B.; Tikhomirova, I.N.; Roslyakov, I.V.; Khayrutdinova, D.; Platonov, V.; Boytsova, O.; Kaimonov, M.; et al. Powder Synthesized from Aqueous Solution of Calcium Nitrate and Mixed-Anionic Solution of Orthophosphate and Silicate Anions for Bioceramics Production. Coatings 2023, 13, 374. https://doi.org/10.3390/coatings13020374
Golubchikov D, Safronova TV, Nemygina E, Shatalova TB, Tikhomirova IN, Roslyakov IV, Khayrutdinova D, Platonov V, Boytsova O, Kaimonov M, et al. Powder Synthesized from Aqueous Solution of Calcium Nitrate and Mixed-Anionic Solution of Orthophosphate and Silicate Anions for Bioceramics Production. Coatings. 2023; 13(2):374. https://doi.org/10.3390/coatings13020374
Chicago/Turabian StyleGolubchikov, Daniil, Tatiana V. Safronova, Elizaveta Nemygina, Tatiana B. Shatalova, Irina N. Tikhomirova, Ilya V. Roslyakov, Dinara Khayrutdinova, Vadim Platonov, Olga Boytsova, Maksim Kaimonov, and et al. 2023. "Powder Synthesized from Aqueous Solution of Calcium Nitrate and Mixed-Anionic Solution of Orthophosphate and Silicate Anions for Bioceramics Production" Coatings 13, no. 2: 374. https://doi.org/10.3390/coatings13020374
APA StyleGolubchikov, D., Safronova, T. V., Nemygina, E., Shatalova, T. B., Tikhomirova, I. N., Roslyakov, I. V., Khayrutdinova, D., Platonov, V., Boytsova, O., Kaimonov, M., Firsov, D. A., & Lyssenko, K. A. (2023). Powder Synthesized from Aqueous Solution of Calcium Nitrate and Mixed-Anionic Solution of Orthophosphate and Silicate Anions for Bioceramics Production. Coatings, 13(2), 374. https://doi.org/10.3390/coatings13020374