1. Introduction
Stirred fermenters (stirred bioreactors) are widely applied in the pharmaceutical industry to produce pharmaceutical compounds. The use of these unit operations has become the leading solution for production of microbial cells at an industrial scale [
1]. Despite the common use of stirred fermenters, the understanding of mixing and hydrodynamic characteristics of these systems is still limited and the optimization of mixing conditions in this equipment has remained a challenging task. In general, a stirred fermenter needs to be designed and operated in order to achieve the following:
Hydrodynamic and mixing characteristics of stirred fermenters depend greatly on the geometrical parameters (e.g., impeller’s type, size, and position as well as the number of impellers) and operating conditions (e.g., impeller’s rotational speed, the medium composition, and microbial cell properties). Various types of impellers can be used in stirred bioreactors to enhance mixing homogeneity, to reduce mixing time and power consumption [
3], and to control the shear conditions and hydrodynamic forces experienced by cells [
4,
5,
6]. The type of impeller is decided upon based on the bioprocess application and mechanical properties of the cells. Conventionally, the Rushton impeller has been widely used in aerated stirred bioreactors due to its high turbulence and gas dispersion capabilities as well as its high gas–liquid mass transfer [
4,
6]. However, it is known that this impeller has a high power number: it creates a non-homogenous turbulence energy dissipation distribution (high values in the vicinity of the impeller and low values in the other zone of the mixing tank) as well as compartmentalization with axial flow barriers [
6]. One type of impeller that has recently drawn attention in stirred bioreactors is referred to as a Segment or Elephant Ear (EE) (because of its blade shape). It typically has three wide blades, and its main characteristics are high solidity and low shear intensity along the impeller blade [
7]. This impeller is particularly considered as a suitable candidate for cultivation of shear-sensitive microorganisms [
4,
8]. Venkat and Chalmers [
9] characterized the flow pattern around this impeller using Particle Tracking Velocimetry experimental techniques. Zhu et al. [
7] studied the performance of EE upward-pumping (EEU) and EE downward-pumping (EED) under both aerated and unaerated conditions experimentally. In the unaerated experiments, the power number of EED was larger than the power number of EEU, and the maximum values of turbulence kinetic energy (TKE) and flow numbers were similar for both EEU and EED. The authors concluded that upward-pumping mode showed some advantages over downward-pumping for aerated cases. Simmons et al. [
10] compared the power and flow characteristics (flow pattern and mixing time) of an EE impeller with two other axial impellers, namely a six-blade pitched blade turbine (PBT) and a B2 hydrofoil operating in an upward-pumping mode and turbulent regime (300–650 RPM) experimentally. They reported that the power number and axial flow numbers for the EE impeller were higher than two other impellers. Particle image velocimetry (PIV) results showed that the global flow fields were similar for all impellers, and the TKE value produced by all impellers reached a similar value. The authors concluded that there was no conclusive proof that the EE impeller generated a lower shear rate than other studied impellers. Of note, their study was limited to single liquid phase flow. Collingnon et al. [
8], however, reported that the EED impeller generated the lowest shear rate among all tested axial impellers when compared at the just-suspended speed. The authors also reported a high suspending capacity of the EE impeller (i.e., microcarrier full suspension was achieved at a very low rotational speed) compared to other studied axial impellers. Bustamante et al. [
4] conducted a study in order to evaluate the shear rate generated by the Rushton impeller compared to an EE impeller in an aerated mixing tank for both Newtonian and non-Newtonian fluids. They observed that, for both types of fluids, the oxygen mass transfer coefficient (
kLa) ranges for the Rushton and the EEU impeller were similar and higher than those gained from the EED impeller. Furthermore, the EE impeller generated lower shear rate values compared to the systems equipped with the Rushton impeller. The authors also concluded that EEU with good oxygen mass transfer and low shear stress could be considered as an appropriate impeller for bioprocesses including shear-sensitive microorganisms.
The application of double or multiple impellers in bioreactors is prevalent in order to eliminate the dead zones, to improve mixing homogeneity, and to shorten mixing time [
6,
11]. Using double or multiple impellers in bioreactors, however, introduces more geometrical parameters to be optimized such as impeller type combination/configuration (all radial, all axial, or a combination of axial and radial impellers), identical or different impeller size, spacing between impellers, and number of impellers [
12]. Depending on the spacing between impellers and the size of the impellers, the intensity of interaction between impellers can vary and different flow hydrodynamics can be generated. Rutherford et al. [
11] classified the flow patterns in a stirred tank equipped with double Rushton impellers as parallel, merging, and diverging flows. The influence of spacing between impellers on the flow pattern of a mixing tank equipped with double-axial impellers was also discussed by Hari-Prajitno et al. [
13]. It has been pointed out in literature that, when there is no significant interaction between impellers, the fluid flow generated by each impeller in a multiple-impeller system is similar to the fluid flow generated by that impeller in a single-impeller system [
12].
Various experimental techniques such as Electrical Resistance Tomography (ERT) [
14], Laser Doppler Velocimetry (LDV) [
3], and Particle Image Velocimetry (PIV) [
7,
10,
15] have been used in order to investigate the performance of stirred bioreactors. These experimental methods are extremely time consuming to fully characterize the fluid flow in bioreactors. Although these sophisticated experimental methods can provide a general understanding of the mixing characteristics in the stirred bioreactors, capturing critical fluid-flow data such as the local and temporal heterogeneity of turbulence, shear and normal stress distribution, and hydrodynamic forces acting on cells is challenging or even impossible with the current development of experimental systems. Furthermore, the application of experimental techniques to characterize large-scale bioreactors is not usually practical due to the lack of optical accessibility [
16]. In addition, trial and error experiments can be costly, especially when the biological material is expensive and can result in producing a large amount of biological waste. Therefore, simulation methods have recently become increasingly popular in the investigation of stirred bioreactors. Simulation techniques can provide spatially and time-resolved information, which cannot be obtained conveniently through experiments. One of the numerical approaches that has been successfully used in simulation of bioreactors is referred to as Computational Fluid Dynamics (CFD). CFD has shown its capability in predicting the fluid flow and underlying phenomena happening in stirred bioreactors in several studies [
16,
17,
18,
19]. It should be noted that, despite the popularity of CFD in the simulation of biotechnological apparatus, it needs to be validated by experimental data as there are some assumptions and simplified models in CFD. Depending on the desirable parameters to study, numerical simulations can be performed for a single phase [
20], two phases [
21], or three phases [
22]. Kaiser et al. [
20] used single-phase CFD simulations in order to compare the flow pattern of the 2-litre single-use bioreactor and its reusable counterpart cell-culture bioreactors. The bioreactors were equipped with a double impeller (the lower impeller was a Rushton impeller and the upper impeller was a Segment impeller) operating in downward-pumping mode. The impellers had almost identical diameter, and the distance between them was 1.25 times the impeller’s diameter. The CFD simulation results showed that the fluid velocity profiles and turbulence distributions were very similar in both bioreactors. In their simulations for both bioreactors, the Segment impeller showed a downward axial flow pattern and the Rushton impeller discharged the flow in the radial direction.
The objective of this study is to demonstrate the application of CFD in investigating the effect of the impeller configuration (a design parameter) and impeller rotational speed (an operational parameter) on the hydrodynamic behaviour and mixing performance of a stirred fermenter equipped with a double impeller. The hydrodynamic behaviour of a bioreactor equipped with the selected impeller configurations has rarely been investigated in literature. In this study, velocity profiles, stress generated by impellers due to the turbulence and velocity gradient, and mixing time were quantified to compare various CFD simulations.
2. Experimental Setup and Measurements
The stirred fermenter under investigation in this study was a 0.02-m
3 Sartorius fermenter available at the Sanofi Pasteur laboratory. This fermenter is used for Tetanus fermentations. Two different double-impeller configurations were used in the current study. One impeller configuration included a Rushton and a Segment impeller (i.e., Ruston was the lower impeller, and Segment was the upper impeller), and another impeller configuration had two Segment impellers. The Segment impellers were manufactured by bbi-biotech GmbH. In all experiments, the total liquid volume was 0.015 m
3, resulting in the initial liquid height to be 1.14 times the fermenter diameter (
LH = 1.14
T). As in Tetanus fermentations, the density and viscosity of the medium is comparable with water. Therefore, room-temperature water was used in all experiments. The dimensions of the fermenter vessel and impellers are summarized in
Table 1 and are shown in
Figure 1a,b. In all experimental cases, the direction of impeller rotation was selected to generate an upward-pumping flow in the fermenters (i.e., clockwise as shown in
Figure 1c,d). The submergence of the upper impeller in all cases was selected to guarantee that the vortex was not formed, and there was no entrainment of air for all operating conditions tested in this study.
In the current study, six cases were considered to analyze the influence of impeller configuration and RPM on the hydrodynamic behaviour and mixing performance of a laboratory-scale fermenter. The experimental cases are summarized in
Table 2.
The torque value for each case was measured experimentally by using a rotary sensor (manufactured by S. Himmelstein) attached to the shaft. The measured experimental torque values were used to calculate power values (, where is torque and is impeller speed). The obtained power values were used in order to validate the CFD models. The experimental measurements were repeated three times to assure that measured torque values are reproducible.
3. Simulation Methodology and CFD Model Validation
In the current study, the Reynolds–Average Navier–Stokes (RANS) equations were solved to simulate the fluid flow in the stirred fermenter by ANSYS
® FLUENT, Release 16.2 (ANSYS, Inc, Canonsburg, PA 15317, USA). The liquid was assumed to be a Newtonian fluid with properties similar to water.
where
,
,
,
,
, and
are the fluid density, fluid average velocity, pressure, gravitational acceleration, viscose stress tensor, and Reynolds–Stress tensor, respectively. To simulate the motion of impellers, the Multiple Reference Frame (MRF) method was employed in CFD simulations.
As seen in
Table 3, calculating the Reynolds number (
) showed that the liquid flow inside the tank was in the turbulent regime in all simulation cases. Therefore, the
turbulence model was used to describe the Reynolds stress tensor.
Other CFD settings and parameters selected in all simulation cases are summarized in
Table 4. Six simulation cases similar to the experimental cases were performed in this study. All simulations were performed on High-Performance Computing Virtual Laboratory (HPCVL) Canada.
Initially, the influence of grid numbers on the CFD results (grid independence) was tested. Two rotating zones around the two impellers were defined with the finer mesh sizes. The grid size around the impellers was 1/3 of the grid size in other regions of the simulation domain. Three different simulation cases with 700,000, 1 million, and 1.3 million mesh elements (i.e., unstructured tetrahedral elements) were simulated to find the optimal mesh size (i.e., number of mesh elements). To obtain the desired mesh element sizes and numbers, the sizing function in the ANSYS meshing tool was used. The velocity magnitude on a line crossing the fermenter at the Segment impeller height (
Z = 0.187 m) was used as a parameter to evaluate the mesh quality. The comparison between cases was performed when a steady-state condition was achieved. Tracking the values of torque obtained from simulations demonstrated that the torque value did not change considerably between 10 to 15 s of simulations. This confirmed that the steady-state condition was reached at around 10 s of simulations. As seen in
Figure 2, the instantaneous velocity magnitude values obtained from simulations with 1 and 1.3 million mesh elements showed similar trends and were relatively close, with around a 10% difference in their averaged values. This small discrepancy between results might be tolerated for the sake of shorter computational time obtained from the simulation with 1 million mesh elements. The simulation with 700,000 mesh elements showed a different trend in comparison with other simulation cases. Comparing the velocity magnitude values obtained from simulations with 700,000 with those obtained from simulations with 1 and 1.3 million mesh elements showed relatively high differences in their values. Therefore, 1 million mesh elements were used in all simulations in the current study, as it provided a compromise between computational time and simulation accuracy.
As mentioned previously, in this study, the CFD models were validated by comparing the power values (
) measured experimentally with power values obtained from the CFD models for various simulation cases as tabulated in
Table 5.
As it can be seen, a relatively close agreement between the simulation results and experimental data is observed. This suggests that the CFD model with the selected input parameters and settings could replicate the phenomena happening in the experiments. The observed error in
Table 5 can be mainly attributed to the selected mesh size. As previously mentioned, 1 million mesh elements were chosen as an optimal mesh element number as it provided relatively close results to the fine mesh size (1.3 million mesh elements) at a reasonable computational time. Using the finer mesh size in the CFD simulations may decrease the discrepancy between the simulations and experiments at the cost of higher computational time. In addition, in this study, the
turbulence model (the most widely used turbulence model in literature) was used to simulate stirred bioreactors due to its numerical convenience [
16,
17,
20,
23,
24]. However, as mentioned by Singh et al. [
25] in the simulation of a mixing tank with Rushton impeller, the
turbulence model may adversely affect the model accuracy. Aubin et al. [
26] found that using a more sophisticated turbulence model such as Reynolds stress model in a CFD simulation of a stirred tank had a slight effect on turbulent kinetic energy and mean flow compared to the simulation case using the
turbulence model. The authors also mentioned the CFD convergence difficulties when using the Reynolds stress model. Therefore, using more sophisticated turbulence models such as the Reynolds stress model may not help to reduce the error. More accurate modeling approaches such as direct numerical simulation (DNS) or large eddy simulations (LES) at a considerably higher computational time can be considered to reduce the observed error.
5. Conclusions
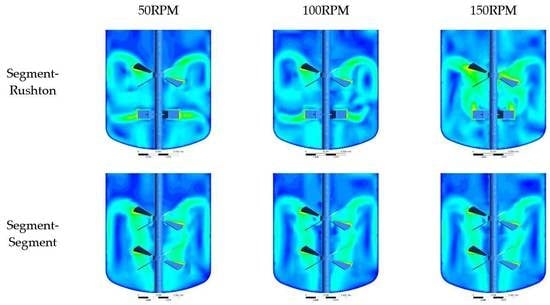
In this study, CFD simulations were applied to comprehensively investigate the influence of impeller configuration and RPM on the mixing performance and hydrodynamic behaviour of a laboratory-scale bioreactor operating in the turbulent regime. Two different impeller configurations (Segment–Segment and Segment–Rushton) and three different RPM values (50, 100, and 150) were used in the CFD simulations. To validate the CFD model, the power values obtained from the CFD models were compared with the experimentally measured power values. The power values were in close agreement. This indicated that the CFD models could represent the experimentations accurately. For simulations with the Segment–Rushton impeller configuration, it was observed that, at the lowest RPM value (50), two impellers operated independently with minimum interaction. The Rushton and Segment impellers discharged the flow in radial and axial directions, respectively. However, as RPM was increased, the intensity of the interaction between the impellers increased. At the highest RPM value, the high axial flow generated by the Segment impeller altered the fluid hydrodynamics around the Rushton impeller considerably and the Rushton impeller did not operate as a radial impeller. For simulations with the Segment–Segment impeller configuration, two impellers interacted and two circulation loops were created in the fermenter tank in all cases. CFD simulations demonstrate that, at a constant RPM, the Segment–Rushton impeller configuration had higher and values, average strain rate magnitude, average values, and lower values compared to the Segment–Segment impeller configuration. It then could be concluded that the Segment–Rushton impeller configuration may not be suitable for cultivation of shear-sensitive microorganisms. Calculating the mixing time and energy consumption showed that, at low RPM values (50 and 100), the Segment–Segment impeller configuration had a better performance. However, at the highest RPM, the Segment–Rushton impeller had a shorter mixing time and energy consumption, which could be attributed to the high turbulent flow generated by this type of impeller configuration. The CFD simulation results also showed that increasing the RPM increased the values, average strain rate magnitude, and average values and shortened the mixing time regardless of the impeller configuration employed.
This study shows that CFD can be used as a valuable tool to obtain detailed information regarding the stirred bioreactors which might otherwise be challenging or impossible to obtain experimentally. With the rapid advancement in computational facilities, more accurate but computationally demanding simulation techniques such as LES and DNS can be used in future studies to simulate stirred bioreactors. These approaches can provide a more comprehensive understanding of the system.