Chimera Diimine Ligands in Emissive [Cu(P^P)(N^N)][PF6] Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of 6-Cl-6′-Mebpy
2.2. Synthesis and Characterization of [Cu(POP)(6-Cl-6′-Mebpy)][PF6] and [Cu(xantphos)(6-Cl-6′-Mebpy)][PF6]
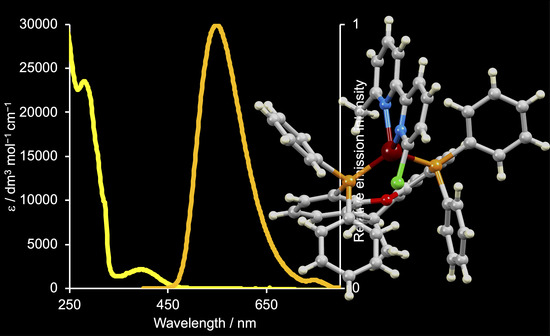
2.3. Electrochemical and Photophysical Properties of [Cu(POP)(6-Cl-6′-Mebpy)][PF6] and [Cu(xantphos)(6-Cl-6′-Mebpy)][PF6]
3. Materials and Methods
3.1. General
3.2. 6-Chloro-6′-methyl-2,2′-bipyridine (6-Cl-6′-Mebpy)
3.3. [Cu(POP)(6-Cl-6′-Mebpy)][PF6]
3.4. [Cu(xantphos)(6-Cl-6′-Mebpy)][PF6]
3.5. Crystallography
3.6. [Cu(POP)(6-Cl-6′-Mebpy)][PF6]
3.7. [Cu(xantphos)(6-Cl-6′-Mebpy)][PF6]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lighting the Future. Available online: https://ec.europa.eu/digital-single-market/en/lighting-future (accessed on 27 March 2020).
- Costa, R.D. (Ed.) Light-Emitting Electrochemical Cells: Concepts, Advances and Challenges; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Fresta, E.; Costa, R.D. Beyond traditional light-emitting electrochemical cells—A review of new device designs and emitters. J. Mater. Chem. C 2017, 5, 5643–5675. [Google Scholar] [CrossRef]
- Kamer, P.C.J.; van Leeuwen, P.W.N.M.; Reek, J.N.H. Wide Bite Angle Diphosphines: Xantphos Ligands in Transition Metal Complexes and Catalysis. Acc. Chem. Rev. 2001, 34, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Buckner, M.T.; McMillin, D.R. Photoluminescence from copper(I) complexes with low-lying metal-to-ligand charge transfer excited states. J. Chem. Soc. Chem. Commun. 1978, 759–761. [Google Scholar] [CrossRef]
- Rader, R.A.; McMillin, D.R.; Buckner, M.T.; Matthews, T.G.; Casadonte, D.J.; Lengel, R.K.; Whittaker, S.B.; Darmon, L.M.; Lytle, F.E. Photostudies of 2,2′-bipyridine bis(triphenylphosphine)copper(1+), 1,10-phenanthroline bis(triphenylphosphine)copper(1+), and 2,9-dimethyl-1,10-phenanthroline bis(triphenylphosphine)copper(1+) in solution and in rigid, low-temperature glasses. Simultaneous multiple emissions from intraligand and charge-transfer states. J. Am. Chem. Soc. 1981, 103, 5906–5912. [Google Scholar] [CrossRef]
- Czerwieniec, R.; Leitl, M.J.; Homeier, H.H.H.; Yersin, H. Cu(I) complexes—Thermally activated delayed fluorescence. Photophysical approach and material design. Coord. Chem. Rev. 2016, 325, 2–28. [Google Scholar] [CrossRef]
- Bergmann, L.; Zink, D.M.; Bauman, T.; Volz, D.; Bräse, S. Metal-Organic and Organic TADF Materials: Status, Challenges and Characterization. Top. Curr. Chem. 2016, 374, 22. [Google Scholar] [CrossRef]
- Elie, M.; Gaillard, S.; Renaud, J.-L. Light-Emitting Electrochemical Cells; Costa, R.D., Ed.; Springer International Publishing: Cham, Swizerland, 2017; pp. 287–327. [Google Scholar] [CrossRef]
- Keller, S.; Constable, E.C.; Housecroft, C.E.; Neuburger, M.; Prescimone, A.; Longo, G.; Pertegás, A.; Sessolo, M.; Bolink, H.J. [Cu(bpy)(P^P)]+ containing light-emitting electrochemical cells: Improving performance through simple substitution. Dalton Trans. 2014, 43, 16593–16596. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.; Pertegás, A.; Longo, G.; Martínez, L.; Cerdá, J.; Junquera-Hernández, J.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E.; Ortí, E.; et al. Shine bright or live long: Substituent effects in [Cu(N^N)(P^P)]+-based light-emitting electrochemical cells where N^N is a 6-substituted 2,2′-bipyridine. J. Mater. Chem. C 2016, 4, 3857–3871. [Google Scholar] [CrossRef] [Green Version]
- Alkan-Zambada, M.; Keller, S.; Martínez-Sarti, L.; Prescimone, A.; Junquera-Hernández, J.M.; Constable, E.C.; Bolink, H.J.; Sessolo, M.; Ortí, E.; Housecroft, C.E. [Cu(P^P)(N^N)][PF6] compounds with bis(phosphane) and 6-alkoxy, 6-alkylthio, 6-phenyloxy and 6-phenylthio-substituted 2,2′-bipyridine ligands for light-emitting electrochemical cells. J. Mater. Chem. C 2018, 6, 8460–8471. [Google Scholar] [CrossRef] [Green Version]
- Fresta, E.; Volpi, G.; Milanesio, M.; Garino, C.; Barolo, C.; Costa, R.D. Novel Ligand and Device Designs for Stable Light-Emitting Electrochemical Cells Based on Heteroleptic Copper(I) Complexes. Inorg. Chem. 2018, 57, 10469–10479. [Google Scholar] [CrossRef]
- Keller, S.; Prescimone, A.; Bolink, H.J.; Sessolo, S.; Longo, G.; Martínez-Sarti, L.; Junquera-Hernández, J.M.; Constable, E.C.; Ortí, E.; Housecroft, C.E. Luminescent copper(I) complexes with bisphosphane and halogen-substituted 2,2′-bipyridine ligands. Dalton Trans. 2018, 47, 14263–14276. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.D.; Tordera, D.; Ortí, E.; Bolink, H.J.; Schönle, J.; Graber, S.; Housecroft, C.E.; Constable, E.C.; Zampese, J.A. Copper(I) Complexes for Sustainable Light-Emitting Electrochemical Cells. J. Mater. Chem. 2011, 21, 16108–16118. [Google Scholar] [CrossRef]
- Zambach, W.; Quaranta, L.; Massol-Frieh, C.; Trah, S.; Stierli, D.; Pouliot, M.; Nebel, K. Novel Microbiocides. Patent WO 2013026866A2, 28 February 2013. [Google Scholar]
- Sánchez-Castellanos, M.; Flores-Leonar, M.M.; Mata-Pinzón, Z.; Laguna, H.G.; García-Ruiz, K.M.; Rozenel, S.S.; Ugalde-Saldívar, V.M.; Moreno-Esparza, R.; Pijpers, J.J.H.; Amador-Bedolla, C. Theoretical exploration of 2,2′-bipyridines as electro-active compounds in flow batteries. Phys. Chem. Chem. Phys. 2019, 21, 15823–15832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, F.; Graber, S.; Baumgartner, Y.; Häussinger, D.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. The effects of introducing sterically demanding aryl substituents in [Cu(N^N)(P^P)]+ complexes. Dalton Trans. 2017, 46, 6379–6391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, S.; Alkan-Zambada, M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Extended π-Systems in Diimine Ligands in [Cu(P^P)(N^N)][PF6] Complexes: From 2,2′-Bipyridine to 2-(Pyridin-2-yl)quinoline. Crystals 2020, 10, 255. [Google Scholar] [CrossRef] [Green Version]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Keller, S.; Brunner, F.; Junquera-Hernández, J.M.; Pertegás, A.; La-Placa, M.-G.; Prescimone, A.; Constable, E.C.; Bolink, H.J.; Ortí, E.; Housecroft, C.E. CF3 Substitution of [Cu(P^P)(bpy)][PF6] complexes: Effects on Photophysical Properties and Light-emitting Electrochemical Cell Performance. ChemPlusChem 2018, 83, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Brunner, F.; Babaei, A.; Pertegás, A.; Junquera-Hernández, J.M.; Prescimone, A.; Constable, E.C.; Bolink, H.J.; Sessolo, M.; Ortí, E.; Housecroft, C.E. Phosphane tuning in heteroleptic [Cu(N^N)(P^P)]+ complexes for light-emitting electrochemical cells. Dalton Trans. 2019, 48, 446–460. [Google Scholar] [CrossRef] [Green Version]
- Alkan-Zambada, M.; Hu, X. Cu Photoredox Catalysts Supported by a 4,6-Disubstituted 2,2′-Bipyridine Ligand: Application in Chlorotrifluoromethylation of Alkenes. Organometallics 2018, 21, 3928–3935. [Google Scholar] [CrossRef] [Green Version]
- MacPhee, J.A.; Panaye, A.; Dubois, J.-E. Steric Effects—I: A Critical Examination of the Taft Steric Parameter – ES. Definition of a revised, broader and homogeneous scale. Extension to highly congested alkyl groups. Tetrahedron 1978, 34, 3553–3562. [Google Scholar] [CrossRef]
- Belot, V.; Farran, D.; Jean, M.; Albalat, M.; Vanthuyne, N.; Roussel, C. Steric Scale of Common Substituents from Rotational Barriers of N-(o-Substituted aryl)thiazoline-2-thione Atropisomers. J. Org. Chem. 2017, 82, 10188–10200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrés-Tomé, I.; Fyson, J.; Baiao Dias, F.; Monkman, A.P.; Iacobellis, G.; Coppo, P. Copper(I) complexes with bipyridyl and phosphine ligands: A systematic study. Dalton Trans. 2012, 41, 8669–8674. [Google Scholar] [CrossRef] [PubMed]
- Hofbeck, T.; Monkowius, U.; Yersin, H. Highly Efficient Luminescence of Cu(I) Compounds: Thermally Activated Delayed Fluorescence Combined with Short-Lived Phosphorescence. J. Am. Chem. Soc. 2015, 137, 399–404. [Google Scholar] [CrossRef]
- Kubas, G.J.; Monzyk, B.; Crumbliss, A.L. Tetrakis(acetonitrile)copper(I) hexafluorophosphate. Inorg. Synth. 1979, 19, 90–92. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. Superflip—A Computer Program for the Solution of Crystal Structures by Charge Flipping in Arbitrary Dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef] [Green Version]
- Palatinus, L.; Prathapa, S.J.; van Smaalen, S. EDMA: A Computer Program for Topological Analysis of Discrete Electron Densities. J. Appl. Cryst. 2012, 45, 575–580. [Google Scholar] [CrossRef]
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J. CRYSTALS version 12: Software for guided crystal structure analysis. J. Appl. Cryst. 2003, 36, 1487. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and Analysis of Crystal Structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]








| Cation | Cu–N/Å | Cu–P/Å | N–Cu–N/o | P–Cu–P/o | P–Cu–N/o |
|---|---|---|---|---|---|
| [Cu(POP)(6-Cl-6′-Mebpy)]+ | 2.103(2), 2.131(3) | 2.2497(11), 2.2774(13) | 78.95(14) | 116.51(5) | 116.38(8), 112.11(8), 120.35(10), 106.79(11) |
| [Cu(xantphos)(6-Cl-6′-Mebpy)]+ | 2.104(3), 2.123(3) | 2.2750(13), 2.2978(13) | 78.52(13) | 120.36(5) | 117.08(11), 105.66(10), 114.44(10), 113.21(11) |
| Compound | E1/2 /V | Epc – Epa/mV | Epca/V | Reference |
|---|---|---|---|---|
| [Cu(POP)(6-Cl-6′-Mebpy)][PF6] | +0.98 | This work | ||
| [Cu(xantphos)(6-Cl-6′-Mebpy)][PF6] | +0.91 | 106 | This work | |
| [Cu(POP)(6,6′-Cl2bpy)][PF6] | +0.98 | 170 | [14] | |
| [Cu(xantphos)(6,6′-Cl2bpy)][PF6] | +0.93 | 90 | [14] | |
| [Cu(POP)(6,6′-Me2bpy)][BF4] | +0.82 b | – c | [26] | |
| [Cu(xantphos)(6,6′-Me2bpy)][PF6] | +0.90 | 150 | [21] | |
| [Cu(POP)(bpy)][PF6] | +0.72 | 110 | [21] | |
| [Cu(xantphos)(bpy)][PF6] | +0.76 | 110 | [21] |
| Compound | λmax/nm (εmax/dm3 mol−1 cm−1) | |
|---|---|---|
| π*←π | MLCT | |
| [Cu(POP)(6-Cl-6′-Mebpy)][PF6] | 294 (18,800), 322 sh (11,500) | 400 (2,160) |
| [Cu(xantphos)(6-Cl-6′-Mebpy)][PF6] | 283 (23,370), 307 sh (15,200), 322 sh (9,500) | 400 (2,160) |
| Compound | Solution (CH2Cl2, De-Aerated, 1.0 × 10−5 mol dm−3) | Powder | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| λexc/nm | λmaxem/nm | PLQY/% | τ/ns | λexc/nm | λmaxem/nm | PLQY/% | τ/μsa | τ(1)/ μs (A1) | τ(2)/ μs (A2) | |
| [Cu(POP)(6-Cl-6′-Mebpy)][PF6] | 400 | 593 | 5.5 | 1300 | 400 | 558 | 24 | 4.8 | 6.0 (0.66) | 1.8 (0.25) |
| [Cu(xantphos)(6-Cl-6′-Mebpy)][PF6] | 400 | 582 | 1.4 | 420 | 400 | 548 | 16 | 4.0 | 6.0 (0.49) | 1.3 (0.37) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, M.; Brunner, F.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Chimera Diimine Ligands in Emissive [Cu(P^P)(N^N)][PF6] Complexes. Inorganics 2020, 8, 33. https://doi.org/10.3390/inorganics8050033
Meyer M, Brunner F, Prescimone A, Constable EC, Housecroft CE. Chimera Diimine Ligands in Emissive [Cu(P^P)(N^N)][PF6] Complexes. Inorganics. 2020; 8(5):33. https://doi.org/10.3390/inorganics8050033
Chicago/Turabian StyleMeyer, Marco, Fabian Brunner, Alessandro Prescimone, Edwin C. Constable, and Catherine E. Housecroft. 2020. "Chimera Diimine Ligands in Emissive [Cu(P^P)(N^N)][PF6] Complexes" Inorganics 8, no. 5: 33. https://doi.org/10.3390/inorganics8050033
APA StyleMeyer, M., Brunner, F., Prescimone, A., Constable, E. C., & Housecroft, C. E. (2020). Chimera Diimine Ligands in Emissive [Cu(P^P)(N^N)][PF6] Complexes. Inorganics, 8(5), 33. https://doi.org/10.3390/inorganics8050033