A Quasi-Intramolecular Solid-Phase Redox Reaction of Ammonia Ligands and Perchlorate Anion in Diamminesilver(I) Perchlorate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Efforts to Synthesize of Compound 1 and Its Hydrate(s)
2.2. Phase Transition of Compound 1
2.3. General Spectroscopic Characterization and Correlation Analysis of Compounds 1-M and 1-O
2.4. Perchlorate Anion Modes
2.5. Ammonia Ligand Modes
2.6. Assignation of Vibrational Modes in Compound 1 Polymorphs
2.6.1. Anion Modes
2.6.2. Cation Modes
2.7. UV Spectroscopic Studies of Complex 1
2.8. Non-Isothermal Decomposition of Compound 1 in an Inert Atmosphere and Air
2.9. The Nature of the Final Decomposition Product Formed in the Thermal Decomposition of Compound 1
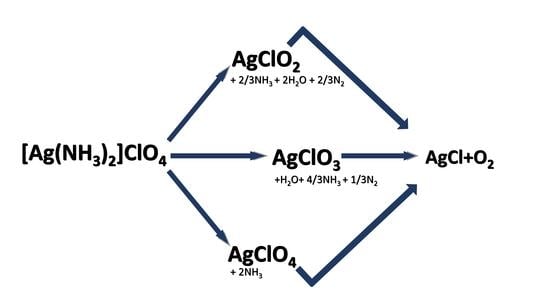
2.10. Redox Interactions in the Thermal Decomposition Steps of Compound 1
2.11. The Nature of Decomposition Intermediates Formed at 240 °C
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sajó, I.E.; Bakos, L.P.; Szilágyi, I.M.; Lendvay, G.; Magyari, J.; Mohai, M.; Szegedi, Á.; Farkas, A.; Jánosity, A.; Klébert, S.; et al. Unexpected Sequential NH3/H2O Solid/Gas Phase Ligand Exchange and Quasi-Intramolecular Self-Protonation Yield [NH4Cu(OH)MoO4], a Photocatalyst Misidentified before as (NH4)2Cu(MoO4)2. Inorg. Chem. 2018, 57, 13679–13692. [Google Scholar] [CrossRef] [PubMed]
- Kótai, L.; Gács, L.; Sajó, I.E. Beliefs and Facts in Permanganate Chemistry—An Overview on the Synthesis and the Reactivity of Simple and Complex Permanganates. Trends Inorg. Chem. 2011, 11, 25–104. [Google Scholar] [CrossRef]
- Kocsis, T.; Magyari, J.; Sajó, I.E.; Pasinszki, T.; Homonnay, Z.; Szilágyi, I.M.; Farkas, A.; May, Z.; Effenberger, H.; Szakáll, S.; et al. Evidence of quasi-intramolecular redox reactions during thermal decomposition of ammonium hydroxodisulfitoferriate(III), (NH4)2[Fe(OH)(SO3)2]·H2O. J. Therm. Anal. Calorim. 2018, 132, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Górska, N.; Mikuli, E.; Kótai, L. Spectroscopic, structural and thermal characterization of crystalline [Cr(OC(NH2)2)6]X3 (X = ClO4, BF4 and Cl) complexes. Eur. Chem. Bull. 2014, 3, 474–481. [Google Scholar]
- Franguelli, F.P.; Barta-Holló, B.; Petruševski, V.M.; Sajó, I.E.; Klébert, S.; Farkas, A.; Bódis, E.; Szilágyi, I.M.; Pawar, R.P.; Kótai, L. Thermal decomposition and spectral characterization of di[carbonatotetraamminecobalt(III)] sulfate trihydrate and the nature of its thermal decomposition products. J. Ther. Anal. Calor. 2020, 57, 13679–13692. [Google Scholar] [CrossRef]
- Sajó, I.; Kótai, L.; Keresztury, G.; Gács, I.; Pokol, G.; Kristóf, J.; Soptrayanov, B.; Petrusevski, V.; Timpu, D.; Sharma, P. Studies on the Chemistry of Tetraamminezinc(II) Dipermanganate ([Zn(NH3)4](MnO4)2): Low-Temperature Synthesis of the Manganese Zinc Oxide (ZnMn2O4) Catalyst Precursor. Helv. Chim. Acta 2008, 91, 1646–1658. [Google Scholar] [CrossRef]
- Solt, H.E.; Nemeth, P.; Mohai, M.; Sajo, I.E.; Klébert, S.; Franguelli, F.P.; Fogaca, L.A.; Pawar, R.P.; Kótai, L. Temperature-limited synthesis of copper manganites along the borderline of the amorphous/crystalline state and their catalytic activity in CO oxidation. ACS Omega 2021, 6, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Kotai, L.; Banerji, K.K.; Sajo, I. An unprecedented-type intramolecular redox reaction of solid tetraamminecopper(2+) bis(permanganate)-[Cu(NH3)4](MnO4)2)—A low- temperature synthesis of copper dimanganese tetraoxide-type (CuMn2O4) nanocrystalline catalyst precursors. Helvetica Chim. Acta 2002, 85, 2316–2327. [Google Scholar] [CrossRef]
- Kotai, L.; Sajo, I.; Jakab, E.; Keresztury, G.; Németh, C.; Gács, I.; Menyhárt, A.; Kristóf, J.; Hajba, L.; Petrusevski, V.M.; et al. Studies on the chemistry of [Cd(NH3)4](MnO4)2. A low temperature synthesis route of the CdMn2O4+x type NOx and CH3SH sensor. Z. Anorg. Allgemenien Chem. 2012, 638, 177–186. [Google Scholar] [CrossRef]
- Buck, R.P.; Singhadeja, S.; Roghers, L.B. Ultraviolet Absorption Spectra of Some Inorganic Ions In Aqueous Solutions. Anal. Chem. 1954, 26, 1240–1242. [Google Scholar] [CrossRef]
- Scagliari, G.; Marangoni, A. Isomorfismo fra perclorati e permanganati. Atti R. Accad. LinceiRend. Sci. FisischeMat. E Nat. Ser. Quinta 1914, 23, 12–14. [Google Scholar]
- Bruni, G.; Levi, G. Gli ammoniacati del sali d’argento I. Gazzetta Chim. Ital. 1916, 46, 17–41. [Google Scholar]
- Fox, B.S.; Beyer, M.K.; Bondybey, V.E. Coordination Chemistry of Silver Cations. J. Am. Chem. Soc. 2002, 124, 13613–13623. [Google Scholar] [CrossRef] [PubMed]
- Nockeman, P.; Meyer, G. [Ag(NH3)2]ClO4—Kristallstrukturen, Phasenumwandlung, Schwingungsspektren. Z. Anorg. Allgemenien Chem. 2002, 628, 1636–1640. [Google Scholar] [CrossRef]
- Mitscherlich, E. Ueber die Mangansaeure, Uebermangansaeure, Ueberchlorsaeure und die Salze dieser Saeuren. Ann. Physik Ser. 1832, 25, 287–302. [Google Scholar] [CrossRef]
- Miles, M.G.; Patterson, J.H.; Hobbs, C.W.; Hopper, M.J.; Overend, J.; Tobias, R.S. Raman and infrared spectra of isosteric diammine and dimethyl complexes of heavy metals. Normal-coordinate analysis of (X3Y2)2Z ions and molecules. Inorg. Chem. 1968, 9, 1721–1729. [Google Scholar] [CrossRef]
- Ephraim, F. Ueber die Natur der Nebenvalenzen. Berichte 1918, 51, 706–710. [Google Scholar]
- Shoeib, T.; Milburn, R.K.; Koyanagi, G.K.; Lavrov, V.V.; Bohme, D.K.; Siu, K.W.M.; Hopkinson, A.C. A study of complexes Mg(NH3)n+· and Ag(NH3)n+, where n = 1–8: Competition between direct coordination and solvation through hydrogen bonding. Int. J. Mass Spectrom. 2000, 201, 87–100. [Google Scholar] [CrossRef]
- Holland, P.M.; Castleman, A.W. The thermochemical properties of gas-phase transition metal ion complexes. J. Chem. Phys. 1982, 76, 4195–4205. [Google Scholar] [CrossRef]
- Bruni, G.; Levi, G. Gli ammoniacati del sali d’argento III. Gazzetta Chim. Ital. 1917, 47, 259–272. [Google Scholar]
- Rossini, F.D. Selected Values of Chemical Thermodynamic Properties; Technical Notes Series I; US Government Printing Office: Washington, DC, USA, 1969. [Google Scholar]
- Pejov, L.; Petruševski, V.M. Fourier transform infrared study of perchlorate ((ClO4−)-Cl-35 and (ClO4−)-Cl-37) anions isomorphously isolated in potassium permanganate matrix. Vibrational anharmonicity and pseudo-symmetry effects. J. Phys. Chem. Solids 2002, 63, 1873–1881. [Google Scholar] [CrossRef]
- Fogaca, L.A.; Kováts, E.; Németh, G.; Kamarás, K.; Béres, K.A.; Németh, P.; Petrusevski, V.; Bereczki, L.; Holló, B.B.; Sajó, I.E.; et al. A solid-phase quasi-intramolecular redox reaction of [Ag(NH3)2]MnO4:an easy way to prepare pure AgMnO2. Inorg. Chem. 2021, 60, 3749–3760. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.B.; May, N.V.; Bombicz, P.A. An unknown component of a selective and mild oxidant: Structure and oxidative ability of a double salt-type complex having κ1O- coordinated permanganate anions and three- and four-fold coordinated silver cations. RSC Adv. 2019, 9, 28387–28398. [Google Scholar] [CrossRef] [Green Version]
- Bereczki, L.; Fogaça, L.A.; Dürvanger, Z.s.; Harmat, V.; Kamarás, K.; Németh, G.; Barta Holló, B.; Petruševski, V.M.; Bódis, E.; Farkas, A.; et al. Dynamic disorder in the high-temperature polymorph of bis[diamminesilver(I)] sulfate—reasons and consequences of simultaneous ammonia release from two different polymorphs. J. Coord. Chem. 2021, in press. [Google Scholar]
- Solymosi, F. The Thermal Stability and Some Physical Properties of Silver Chlorite, Chlorate and Perchlorate. Z. Phys. Chem. 1968, 57, 1–18. [Google Scholar] [CrossRef]
- Bruni, G.; Levi, G. Acido chloroso e cloriti. Gazz. Chim. Ital. 1915, 1915, 161–179. [Google Scholar]
- Udupa, M.R. Thermal behaviour of morpholinium perchlorate. Thermoch. Acta 1980, 38, 241–243. [Google Scholar] [CrossRef]
- Tarasenkov, D.N.; Bogosovskaya, A.V. Vapor pressure of binary mixtures: PbCl2 + AgCl, Zh. Obshch. Khim. 1935, 5, 1687–1689. [Google Scholar]
- Grinberg, A.A.; Varshavskii, Y.S. The frequency of coordinated ammonia deformation mode and its relationship with the chemical properties of transition metal ammonia complexes. Primenenie Molekulyarnoi Spektroskopii v Khimii, Sbornik Dokladov., Akad. Nauk SSSR, Sibirskoe Otdelenie, Inst. Fiz., Izd. Nauka. 1966. [Google Scholar]
- Waechter, A. Ueber die chlorsauren Salze. J. Praktische Chem. 1843, 1834, 321–334. [Google Scholar] [CrossRef]
- Mitscherlich, C.G. Über die Verbindungen des Quecksilbers. Ann. Phys. Chem. 1827, 9, 387–415. [Google Scholar] [CrossRef]
- Maass, G.; Jander, G. Die Grundlagen der Chemie in wasserfreier Essigsäure. In Fortschritte Der Chemischen Forschung; Springer: Berlin, Germany, 1953; Volume 2. [Google Scholar]
- Majzik, E.; Franguelli, F.P.; Lendvay, G.; Trif, L.; Németh, C.s.; Farkas, A.; Klébert, S.; Fogaça, L.A.; Szilágyi, I.M.; Kótai, L. Vibrational spectroscopy of dimethylammonium paratungstate-B hydrates. Z. Anorg. Allgem. Chem. 2021. [Google Scholar] [CrossRef]















| IR, cm−1 (298 K) | Raman Shift, cm−1 (532 nm Excitation), Our Measurement | Raman Shift, cm−1 | Assignation | |||||
|---|---|---|---|---|---|---|---|---|
| Our (1-O) | [16] | [14] | 123 K (1-M) | 223 K (1-O) | 298 K (1-O) | aq. soln. [16] | solid [14] | |
| 933 | 940 | - | 929 | - | 933 | 934 | 926 | ν1(A1) (R) |
| 450 | 432 | - | 482,465 sh, 462, 452 | - | 459 | 460 | 460 | ν2(E) (R) |
| 1073 sh, 1053 | 1090 | 1084 | 1109–1044 * | - | 1082, 1044 | 1107 | - | ν3(F2) (IR, R) |
| 615 | 626 | 626 | 624, 612 | 625 | 625 | 629 | - | ν4(F2) (IR, R) |
| 2050–1950 wide | - | - | - | - | - | - | - | ν1 + ν3a,b,c and 2ν3a,b,c |
| - | - | 1109–1044 * | - | 1080 wide | - | - | ν2a,b + ν4a,b,c | |
| - | - | 919, 914 | 915 sh, 910 | 915–910 wide | - | - | 2ν2a,b | |
| - | - | 1259 | 1263, 1239 | 1263, 1239, 1219 | - | - | 4ν4a,b,c | |
| IR | Raman | Assignation | |||||
|---|---|---|---|---|---|---|---|
| Our, 1-O, 298K | [14] | [16] | Our, 1-O, 298K | Our, 1-M, 123K | [14] | aq. solution [16] | |
| 3364 | 3360 | 3370 | 3389, 3362 | - | νas(NH) | ||
| 3285 | 3285 | 3290 | 3297 | 3299, 3291 | 3293 | νs(NH) | |
| 3193, 3182 | 3182 | 3185 | 3185 | 3191, 3183 | 3203 | 2δas(NH) | |
| 2439 | 2δs(NH) | ||||||
| 1610 | 1612 | 1625, 1610 | 1658, 1634 | δas(NH) | |||
| 1351 | - | - | - | - | - | - | 2ρ(NH) |
| 1213, 1238 | 1250 | 1244 | 1259 | 1219 | 1219 | 1223 | δs(NH) |
| 835 | 814 | - | - | ||||
| 653 | - | 650 | 658 | 679, 666 | 679, 666 | ρ(NH) | |
| 430 | νas(AgN) | ||||||
| - | 395 * | 397 | 397 | ||||
| - | 379 * | 383, 373 | 372 | νs(AgN) | |||
| - | 269 | ||||||
| - | 255 | 243 | 240 | δ(NAgN) | |||
| 196 | - | ||||||
| 136 | Lattice, δ(NAgO), AgO2, 161 AgN2 | ||||||
| 67 | Lattice? |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fogaça, L.A.; Bereczki, L.; Petruševski, V.M.; Barta-Holló, B.; Franguelli, F.P.; Mohai, M.; Béres, K.A.; Sajó, I.E.; Szilágyi, I.M.; Kotai, L. A Quasi-Intramolecular Solid-Phase Redox Reaction of Ammonia Ligands and Perchlorate Anion in Diamminesilver(I) Perchlorate. Inorganics 2021, 9, 38. https://doi.org/10.3390/inorganics9050038
Fogaça LA, Bereczki L, Petruševski VM, Barta-Holló B, Franguelli FP, Mohai M, Béres KA, Sajó IE, Szilágyi IM, Kotai L. A Quasi-Intramolecular Solid-Phase Redox Reaction of Ammonia Ligands and Perchlorate Anion in Diamminesilver(I) Perchlorate. Inorganics. 2021; 9(5):38. https://doi.org/10.3390/inorganics9050038
Chicago/Turabian StyleFogaça, Lara Alexandre, Laura Bereczki, Vladimir M. Petruševski, Berta Barta-Holló, Fernanda Paiva Franguelli, Miklós Mohai, Kende Attila Béres, Istvan E Sajó, Imre Miklós Szilágyi, and Laszlo Kotai. 2021. "A Quasi-Intramolecular Solid-Phase Redox Reaction of Ammonia Ligands and Perchlorate Anion in Diamminesilver(I) Perchlorate" Inorganics 9, no. 5: 38. https://doi.org/10.3390/inorganics9050038
APA StyleFogaça, L. A., Bereczki, L., Petruševski, V. M., Barta-Holló, B., Franguelli, F. P., Mohai, M., Béres, K. A., Sajó, I. E., Szilágyi, I. M., & Kotai, L. (2021). A Quasi-Intramolecular Solid-Phase Redox Reaction of Ammonia Ligands and Perchlorate Anion in Diamminesilver(I) Perchlorate. Inorganics, 9(5), 38. https://doi.org/10.3390/inorganics9050038