Effects of Radio Frequency Pretreatment on Quality of Tree Peony Seed Oils: Process Optimization and Comparison with Microwave and Roasting
Abstract
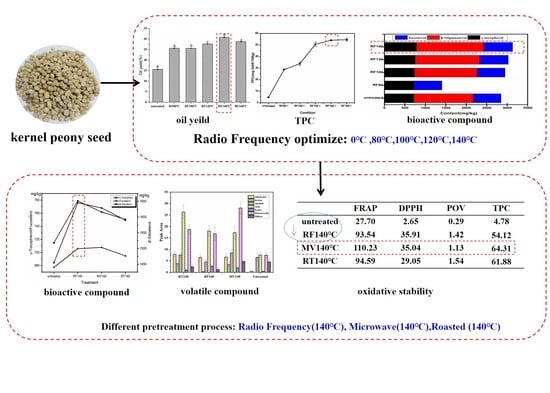
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Pretreatment and Preparation of the Samples
2.3. Cold Pressing
2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5. Transmission Electron Microscopy (TEM)
2.6. Determination of Physical and Chemical Properties
2.7. Determination of the Tocopherols Content
2.8. Determination of Fatty Acids Profile
2.9. Determination of the Phytosterols and Squalene
2.10. Sensory Evaluation
2.11. Determination of Volatile Compounds
2.12. Determination of the Oxidative Stabilities of the Oils
2.13. Determination of the Antioxidant Activity of the Oils
2.14. Statistical Analysis
3. Results
3.1. Different Pretreatments Seed Microstructure (FTIR and TEM)
3.2. Physicochemical Properties of Tree Peony Seed Oil
3.3. Fatty Acid Compositions, Tocopherols and Phytosterols in TPSO
3.4. Sensory Characteristics and Volatile Compounds of TPSO
3.5. Effect of Pretreatment on the Antioxidant Capacities and Oxidative Stabilities of Oils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yu, S.; Du, S.; Yuan, J.; Hu, Y. Fatty acid profile in the seeds and seed tissues of Paeonia L. species as new oil plant resources. Sci. Rep. 2016, 6, 26944. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.L.; Li, Y.Q.; Wang, Z.S.; Sun, G.J.; Qi, X.M.; Mo, H.Z. Physicochemical characteristics and functionality of tree peony (Paeonia suffruticosa Andr.) seed protein. Food Chem. 2018, 240, 980–988. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Contreras, M.D.M.; Verardo, V.; Gomez-Caravaca, A.M.; Xing, C. Integrated profiling of fatty acids, sterols and phenolic compounds in tree and herbaceous peony seed oils: Marker Screening for New Resources of Vegetable Oil. Foods 2020, 9, 770. [Google Scholar] [CrossRef]
- Ning, C.; Jiang, Y.; Meng, J.; Zhou, C.; Tao, J. Herbaceous peony seed oil: A rich source of unsaturated fatty acids and γ-tocopherol. Eur. J. Lipid Sci. Technol. 2015, 117, 532–542. [Google Scholar] [CrossRef]
- Chang, M.; Wang, Z.; Zhang, T.; Wang, T.; Liu, R.; Wang, Y.; Jin, Q.; Wang, X. Characterization of fatty acids, triacylglycerols, phytosterols and tocopherols in peony seed oil from five different major areas in China. Food Res. Int. 2020, 137, 109416. [Google Scholar] [CrossRef]
- Su, J.; Ma, C.; Liu, C.; Gao, C.; Nie, R.; Wang, H. Hypolipidemic activity of peony seed oil rich in alpha-Linolenic, is mediated through inhibition of lipogenesis and upregulation of fatty acid beta-oxidation. J. Food Sci. 2016, 81, H1001–H1009. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, H.; Ma, C.; Lou, Z.; Liu, C.; Tanver Rahman, M.; Gao, C.; Nie, R. Anti-diabetic activity of peony seed oil, a new resource food in STZ-induced diabetic mice. Food Funct. 2015, 6, 2930–2938. [Google Scholar] [CrossRef]
- Wei, X.B.; Xue, J.Q.; Wang, S.L.; Xue, Y.Q.; Lin, H.; Shao, X.F.; Xu, D.H.; Zhang, X.X. Fatty acid analysis in the seeds of 50 Paeonia ostii individuals from the same population. J. Integr. Agric. 2018, 17, 1758–1767. [Google Scholar] [CrossRef] [Green Version]
- Chew, S.C. Cold-pressed rapeseed (Brassica napus) oil: Chemistry and functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Tymczewska, A.; Momot, M.; Włodarczyk, K. Optimization of the microwave treatment of linseed for cold-pressing linseed oil—Changes in its chemical and sensory qualities. LWT Food Sci. Technol. 2020, 126, 109317. [Google Scholar] [CrossRef]
- Li, W.G.; Sun, X.L.; Zu, Y.G.; Zhao, X.H. Optimization peony seed oil extraction process at suitable temperature and physicochemical property analysis. Bull. Bot. Res. 2020, 40, 73–78. [Google Scholar] [CrossRef]
- Prakesh, A.; Dave, V.; Sur, S.; Sharma, P. Vivid techniques of pretreatment showing promising results in biofuel production and food processing. J. Food Process. Eng. 2020, 44, e13580. [Google Scholar] [CrossRef]
- Jiao, Y.; Tang, J.; Wang, Y.; Koral, T.L. Radio-frequency applications for food processing and safety. Annu. Rev. Food Sci. Technol. 2018, 9, 105–127. [Google Scholar] [CrossRef] [Green Version]
- Ghafoor, K.; Ahmed, I.A.M.; Ozcan, M.M.; Al-Juhaimi, F.Y.; Babiker, E.E.; Azmi, I.U. An evaluation of bioactive compounds, fatty acid composition and oil quality of chia (Salvia hispanica L.) seed roasted at different temperatures. Food Chem. 2020, 333, 127531. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Xu, J.; Liu, X.R.; Regenstein, J.M.; Wang, F.J. Roasted tree peony (Paeonia ostii) seed oil: Benzoic acid levels and physicochemical characteristics. Int. J. Food Prop. 2019, 22, 499–510. [Google Scholar] [CrossRef] [Green Version]
- Azadmard-Damirchi, S.; Habibi-Nodeh, F.; Hesari, J.; Nemati, M.; Achachlouei, B.F. Effect of pretreatment with microwaves on oxidative stability and nutraceuticals content of oil from rapeseed. Food Chem. 2010, 121, 1211–1215. [Google Scholar] [CrossRef]
- Ye, M.; Zhou, H.; Hao, J.; Chen, T.; He, Z.; Wu, F.; Liu, X. Microwave pretreatment on microstructure, characteristic compounds and oxidative stability of Camellia seeds. Ind. Crop. Prod. 2021, 161, 113193. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Musa Ozcan, M.; Ghafoor, K.; Babiker, E.E. The effect of microwave roasting on bioactive compounds, antioxidant activity and fatty acid composition of apricot kernel and oils. Food Chem. 2018, 243, 414–419. [Google Scholar] [CrossRef]
- Zhou, X.; Li, R.; Lyng, J.G.; Wang, S.J. Dielectric properties of kiwifruit associated with a combined radio frequency vacuum and osmotic drying. J. Food Eng. 2018, 239, 72–82. [Google Scholar] [CrossRef]
- Zheng, J.; Li, H.; Wang, D.; Li, R.; Wang, S.; Ling, B. Radio frequency assisted extraction of pectin from apple pomace: Process optimization and comparison with microwave and conventional methods. Food Hydrocoll. 2021, 121, 107031. [Google Scholar] [CrossRef]
- Lan, R.; Qu, Y.; Ramaswamy, H.S.; Wang, S. Radio frequency reheating behavior in a heterogeneous food: A case study of pizza. Innov. Food Sci. Emerg. Technol. 2020, 65, 102478. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, L.N.; Wang, X.S.; Gao, J.-Y.; Yi, J.P.; Deng, R.X. Characterization of Paeonia ostii seed and oil sourced from different cultivation areas in China. Ind. Crop. Prod. 2019, 133, 63–71. [Google Scholar] [CrossRef]
- Li, S.S.; Yuan, R.Y.; Chen, L.G.; Wang, L.S.; Hao, X.H.; Wang, L.J.; Zheng, X.C.; Du, H. Systematic qualitative and quantitative assessment of fatty acids in the seeds of 60 tree peony (Paeonia section Moutan DC.) cultivars by GC-MS. Food Chem. 2015, 173, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Zheng, M.; Huang, F.; Liu, C.; Zheng, C. Sinapic acid derivatives in microwave-pretreated rapeseeds and minor components in oils. J. Food Compos. Anal. 2020, 87, 103394. [Google Scholar] [CrossRef]
- National Health Commission. National Standard of the People’s Republic of China; National Health Commission: Beijing, China, 2016.
- Cong, Y.; Cheong, L.Z.; Huang, F.; Zheng, C.; Wan, C.; Zheng, M. Effects of microwave irradiation on the distribution of sinapic acid and its derivatives in rapeseed and the antioxidant evaluation. LWT Food Sci. Technol. 2019, 108, 310–318. [Google Scholar] [CrossRef]
- MPOB. Determination of Carotene Content; Method No. p2.6: 2004; Malaysian Palm Oil Board: Kuala Lumpur, Malaysia, 2005.
- AOCS Official Method Cd18-90. p-Anisidine Value; The American Oil Chemists’ Society: Urbana, IL, USA, 2011. [Google Scholar]
- Xiao, J.; Deng, Q.; Yang, Y.; Xiang, X.; Zhou, X.; Tan, C.; Zhou, Q. Unraveling of the aroma-active compounds in virgin camellia oil (Camellia oleifera Abel) using gas chromatography–mass spectrometry-olfactometry, aroma recombination, and omission studies. J. Agric. Food Chem. 2021, 69, 9043–9055. [Google Scholar] [CrossRef]
- ISO-3656:2002. Animal and Vegetable Fats and Oils. Determination of Ultraviolet Absorbance Expressed as Specific UV Extinction; ISO: Geneva, Switzerland, 2002. [Google Scholar]
- AOCS Official Method Ce 8-89. Determination of Tocopherols and Tocotrienols in Vegetable Oils and Fats by HPLC; The American Oil Chemists’ Society: Urbana, IL, USA, 2009. [Google Scholar]
- Amini Khoozani, A.; Birch, J.; Bekhit, A.E.D.A. Textural properties and characteristics of whole green banana flour produced by air-oven and freeze-drying processing. J. Food Meas. Charact. 2020, 14, 1533–1542. [Google Scholar] [CrossRef]
- Taheri, S.; Brodie, G. Gupta, DFluidisation of lentil seeds during microwave drying and disinfection could prevent detrimental impacts on their chemical and biochemical characteristics. LWT Food Sci. Technol. 2020, 129, 109534. [Google Scholar] [CrossRef]
- Hatamian, M.; Noshad, M.; Abdanan-Mehdizadeh, S.; Barzegar, H. Effect of roasting treatment on functional and antioxidant properties of chia seed flours. NFS J. 2020, 21, 1–8. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Z.; Zhao, Y. Impact of radio frequency, microwaving, and high hydrostatic pressure at elevated temperature on the nutritional and antinutritional components in black soybeans. J. Food Sci. 2015, 80, C2732–C2739. [Google Scholar] [CrossRef]
- Wroniak, M.; Rêkas, A.; Siger, A.; Janowicz, M. Microwave pretreatment effects on the changes in seeds microstructure, chemical composition and oxidative stability of rapeseed oil. LWT Food Sci. Technol. 2016, 68, 634–641. [Google Scholar] [CrossRef]
- Hu, B.; Li, C.; Qin, W.; Zhang, Z.; Liu, Y.; Zhang, Q.; Liu, A.; Jia, R.; Yin, Z.; Han, X.; et al. A method for extracting oil from tea (Camelia sinensis) seed by microwave in combination with ultrasonic and evaluation of its quality. Ind. Crop. Prod. 2019, 131, 234–242. [Google Scholar] [CrossRef]
- Ling, B.; Yang, X.; Li, R.; Wang, S. Physicochemical properties, volatile compounds, and oxidative stability of cold pressed kernel oils from raw and roasted pistachio (Pistacia vera L. Var Kerman). Eur. J. Lipid Sci. Technol. 2016, 118, 1368–1379. [Google Scholar] [CrossRef]
- Suri, K.; Singh, B.; Kaur, A. Impact of microwave roasting on physicochemical properties, maillard reaction products, antioxidant activity and oxidative stability of nigella seed (Nigella sativa L.) oil. Food Chem. 2021, 368, 130777. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Atalay, C. Determination of seed and oil properties of some poppy (Papaver somniferum L.) varieties. Grasas Y Aceites 2006, 57, 169–174. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Jin, J.; Shi, L.K.; Huang, J.H.; Chang, M.; Wang, X.G.; Jin, Q.Z. Gamma tocopherol, its dimmers, and quinones: Past and future trends. Crit. Rev. Food Sci. Nutr. 2020, 60, 3916–3930. [Google Scholar] [CrossRef]
- Thompson, M.D.; Cooney, R.V. The potential physiological role of gammatocopherol in human health: A qualitative review. Nutr. Cancer -Int. J. 2019, 72, 808–825. [Google Scholar] [CrossRef]
- Yu, P.; Yang, Y.; Sun, J.; Jia, X.; Zheng, C.; Zhou, Q.; Huang, F. Identification of volatile sulfur-containing compounds and the precursor of dimethyl sulfide in cold-pressed rapeseed oil by GC-SCD and UPLC-MS/MS. Food Chem. 2022, 367, 130741. [Google Scholar] [CrossRef]
- Xiao, L.; Lee, J.; Zhang, G.; Ebeler, S.E.; Wickramasinghe, N.; Seiber, J.; Mitchell, A.E. HS-SPME GC/MS Characterization of Volatiles in Raw and Dry-Roasted Almonds (Prunus dulcis). Food Chem. 2014, 151, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Tak, J.H.; Ahn, Y.J. Acaricidal activity of Paeonia suffruticosa root bark-derived compounds against Dermatophagoides farinae and Dermatophagoides pteronyssinus (Acari: Pyroglyphidae). J. Agric. Food Chem. 2004, 52, 7857–7861. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Wang, R.; Yuan, Y.H.; Yang, T.K.; Liu, S.Q. Changes in Volatiles of Palm Kernel Oil before and after Kernel Roasting. LWT Food Sci. Technol. 2016, 73, 432–441. [Google Scholar] [CrossRef]
- Chinma, C.E.; Adedeji, O.E.; Etim, I.I.; Aniaka, G.I.; Mathew, E.O.; Ekeh, U.B.; Anumba, N.L. Physicochemical, nutritional, and sensory properties of chips produced from germinated African yam bean (Sphenostylis stenocarpa). LWT Food Sci. Technol. 2021, 136, 110330. [Google Scholar] [CrossRef]
- Diniyah, N.; Alam, M.B.; Lee, S. Antioxidant potential of non-oil seed legumes of Indonesian’s ethnobotanical extracts. Arab. J. Chem. 2020, 13, 5208–5217. [Google Scholar] [CrossRef]
- Ghafoor, K.; Özcan, M.M.; Al-Juhaimi, F.; Babiker, E.E.; Fadimu, G.J. Changes in quality, bioactive compounds, fatty acids, tocopherols, and phenolic composition in oven- and microwave-roasted poppy seeds and oil. LWT Food Sci. Technol. 2019, 99, 490–496. [Google Scholar] [CrossRef]
- Pan, F.; Wen, B.; Wang, X.; Ma, X.; Zhao, J.; Liu, C.; Xu, Y.; Dang, W. Effect of the chemical refining process on perilla seed oil composition and oxidative stability. J. Food Process. Preserv. 2019, 43, e14094. [Google Scholar] [CrossRef]




| Treatment | Acid Value (mg/g) | L | a* | b* | Oil Yield (%) | TPC (mgGAE/100 g) | Total Carotenoids (mg/kg) |
|---|---|---|---|---|---|---|---|
| Untreated | 4.07 ± 0.04 a | 64.81 ± 0.23 a | −4.22 ± 0.13 a | 91.52 ± 0.40 e | 15.57 ± 013 a | 4.78 ± 0.01 a | 0.41 ± 0.02 a |
| RF80 °C | 5.14 ± 0.01 b | 65.20 ± 0.11 a | −1.24 ± 0.13 b | 91.42 ± 0.62 e | 25.59 ± 0.11 b | 28.78 ± 0.21 b | 0.47 ± 0.02 a |
| RF100 °C | 5.21 ± 0.00 b | 65.82 ± 0.44 a | 2.73 ± 0.52 c | 91.19 ± 0.28 e | 25.58 ± 0.07 b | 33.86 ± 1.57 c | 0.47 ± 0.02 a |
| RF120 °C | 6.31 ± 0.03 c | 65.03 ± 0.29 ab | 6.53 ± 0.08 d | 95.48 ± 0.35 d | 27.61 ± 0.05 c | 50.62 ± 2.85 d | 0.47 ± 0.02 a |
| RF140 °C | 6.87 ± 0.01 d | 64.32 ± 0.29 b | 7.67 ± 0.33 e | 104.34 ± 0.33 b | 30.79 ± 0.11 d | 54.12 ± 0.06 e | 0.62 ± 0.15 a |
| MW140 °C | 6.65 ± 0.03 d | 63.48 ± 0.50 c | 11.79 ± 0.33 f | 101.26 ± 0.24 c | 31.88 ± 0.13 e | 64.31 ± 0.01 f | 1.35 ± 0.18 b |
| RT140 °C | 6.91 ± 0.02 d | 62.32 ± 0.27 d | 12.10 ± 0.01 f | 105.69 ± 0.07 a | 30.27 ± 0.21 d | 61.88 ± 0.93 f | 0.71 ± 0.02 a |
| Fatty Acids (%) | |||||||
|---|---|---|---|---|---|---|---|
| Untreated | RF80 °C | RF100 °C | RF120 °C | RF140 °C | MW140 °C | RT 140 °C | |
| C16:0 | 5.640 ± 0.003 a | 5.640 ± 0.091 a | 5.635 ± 0.008 a | 5.648 ± 0.009 ab | 5.695 ± 0.003 ab | 5.646 ± 0.014 ab | 5.731 ± 0.006 b |
| C16:1 | 0.334 ± 0.020 a | 0.385 ± 0.011 ab | 0.365 ± 0.098 ab | 0.424 ± 0.030 ab | 0.381 ± 0.018 ab | 0.463 ± 0.062 b | 0.372 ± 0.034 ab |
| C18:0 | 1.316 ± 0.045 a | 1.275 ± 0.065 a | 1.242 ± 0.008 a | 1.297 ± 0.004 a | 1.282 ± 0.018 a | 1.256 ± 0.000 a | 1.300 ± 0.008 a |
| C18:1 | 22.687 ± 0.078 bc | 22.580 ± 0.296 abc | 22.608 ± 0.004 abc | 22.824 ± 0.093 bc | 22.911 ± 0.018 c | 22.327 ± 0.105 a | 22.590 ± 0.042 abc |
| C18:2 | 24.011 ± 0.380 a | 24.104 ± 0.062 ab | 24.655 ± 0.064 c | 24.291 ± 0.025 abc | 24.414 ± 0.041 bc | 24.426 ± 0.078 bc | 24.258 ± 0.002 ab |
| C18:3 | 46.066 ± 0.501 c | 45.919 ± 0.333 bc | 45.445 ± 0.012 ab | 45.467 ± 0.081 abc | 45.270 ± 0.027 a | 45.883 ± 0.107 bc | 45.750 ± 0.079 abc |
| SFA | 7.288 ± 0.028 a | 7.298 ± 0.166 a | 7.246 ± 0.081 a | 7.368 ± 0.035 a | 7.357 ± 0.033 a | 7.365 ± 0.076 a | 7.403 ± 0.036 a |
| UFA | 92.763 ± 0.042 b | 92.602 ± 0.025 a | 92.707 ± 0.080 ab | 92.585 ± 0.037 a | 92.595 ± 0.033 a | 92.635 ± 0.076 a | 92.598 ± 0.035 a |
| Tocopherols(mg/kg) | |||||||
| α-Tocopherols | 51.76 ± 0.78 d | 49.21 ± 0.79 bcd | 44.92 ± 4.39 ab | 43.64 ± 0.48 a | 45.96 ± 1.44 abc | 49.99 ± 0.20 cd | 44.57 ± 0.97 a |
| γ-Tocopherols | 714.61 ± 4.66 a | 717.64 ± 2.85 ab | 733.58 ± 12.50 bc | 748.56 ± 0.39 cd | 778.76 ± 11.45 e | 761.75 ± 2.96 d | 750.44 ± 1.13 d |
| σ-Tocopherols | 6.40 ± 0.05 b | 6.26 ± 0.03 ab | 6.37 ± 0.05 b | 6.60 ± 0.03 b | 9.51 ± 0.28 c | 5.63 ± 0.49 a | 6.32 ± 0.42 b |
| Total | 785.26 ± 10.28 a | 795.43 ± 24.55 a | 820.24 ± 16.29 a | 781.70 ± 27.64 a | 836.71 ± 13.19 a | 820.02 ± 3.50 a | 803.61 ± 1.68 a |
| Phytosterols(mg/kg) | |||||||
| Squalene | 13.82 ± 0.30 a | 14.45 ± 0.47 ab | 14.36 ± 0.59 ab | 15.22 ± 0.17 bc | 15.62 ± 0.58 cd | 16.58 ± 0.57 d | 15.16 ± 0.25 bc |
| Campesterol | 30.30 ± 0.48 a | 30.63 ± 0.54 ab | 30.98 ± 0.36 ab | 30.78 ± 0.76 ab | 32.02 ± 0.21 bc | 32.87 ± 0.39 c | 33.035 ± 1.23 c |
| γ-Stigmasterol | 8.61 ± 0.85 a | 9.90 ± 0.47 b | 9.73 ± 0.57 b | 10.43 ± 0.43 b | 10.30 ± 0.02 b | 10.62 ± 0.11 b | 10.04 ± 0.24 b |
| β-Sitosterol | 1454.75 ± 4.16 a | 1558.20 ± 4.92 bc | 1532.77 ± 4.74 ab | 1575.57 ± 2.76 bcd | 1645.67 ± 3.03 d | 1628.88 ± 0.43 cd | 1590.28 ± 3.72 bcd |
| Fucosterol | 677.24 ± 0.97 a | 686.41 ± 3.37 abc | 680.31 ± 2.48 ab | 701.03 ± 2.13 cd | 705.75 ± 1.31 d | 707.64 ± 2.22 d | 694.68 ± 1.21 bcd |
| Δ-5-Avenasterol | 117.98 ± 2.29 a | 126.84 ± 2.93 a | 159.79 ± 2.17 b | 164.56 ± 1.90 bc | 167.36 ± 1.51 bc | 171.29 ± 0.98 c | 164.67 ± 1.89 bc |
| Δ-7-Avenasterol | 33.73 ± 1.32 a | 34.69 ± 0.14 ab | 38.04 ± 0.74 c | 36.64 ± 0.48 bc | 37.40 ± 1.15 c | 38.14 ± 0.71 c | 38.18 ± 0.86 c |
| 24-Methylenecycloartan-3β-ol | 119.37 ± 2.26 a | 129.19 ± 0.37 b | 158.25 ± 2.18 c | 178.93 ± 0.98 de | 172.74 ± 1.38 d | 191.16 ± 2.01 f | 182.13 ± 1.85 e |
| Total | 2455.79 ± 44.04 a | 2590.29 ± 32.59 b | 2624.21 ± 32.86 bc | 2713.14 ± 14.16 cd | 2786.85 ± 77.75 d | 2797.14 ± 7.99 d | 2728.16 ± 24.78 cd |
| FRAP (umol TE/100 g) | DPPH (umol TE/100 g) | CD | CT | POV (mmol O2/kg) | P-AV | IP(h) | |
|---|---|---|---|---|---|---|---|
| Untreated | 27.69 ± 1.05 a | 2.65 ± 0.13 a | 1.24 ± 0.005 a | 0.16 ± 0.003 a | 0.29 ± 0.01 a | 1.20 ± 0.11 a | 1.07 ± 0.12 a |
| RF80 °C | 42.56 ± 0.88 b | 3.81 ± 0.31 a | 1.31 ± 0.013 a | 0.16 ± 0.005 a | 0.35 ± 0.01 a | 1.23 ± 0.08 a | 1.21 ± 0.08 a |
| RF100 °C | 79.41 ± 1.86 c | 5.51 ± 0.45 b | 1.49 ± 0.021 a | 0.19 ± 0.009 a | 0.71 ± 0.01 b | 1.20 ± 0.12 a | 2.14 ± 0.08 b |
| RF120 °C | 89.61 ± 0.69 d | 23.82 ± 0.37 c | 1.65 ± 0.025 a | 0.24 ± 0.003 a | 1.07 ± 0.01 c | 1.18 ± 0.05 a | 3.07 ± 0.11 c |
| RF140 °C | 93.53 ± 2.36 e | 35.91 ± 0.15 e | 2.21 ± 0.048 b | 0.23 ± 0.007 a | 1.42 ± 0.01 d | 1.26 ± 0.11 a | 4.33 ± 0.13 d |
| MW140 °C | 110.23 ± 2.04 f | 35.04 ± 0.05 e | 1.35 ± 0.027 a | 0.16 ± 0.002 a | 1.13 ± 0.01 c | 1.49 ± 0.05 a | 4.53 ± 0.15 d |
| RT140 °C | 94.59 ± 1.46 e | 29.05 ± 0.46 d | 1.86 ± 0.008 b | 0.25 ± 0.007 a | 1.54 ± 0.01 d | 1.43 ± 0.03 a | 3.11 ± 0.10 c |
| β-Sitosterol | Fucosterol | Squalene | FRAP | DPPH | TPC | |
|---|---|---|---|---|---|---|
| γ-tocopherol | 0.982 ** | 0.967 ** | 0.895 * | 0.900 * | 0.965 ** | 0.846 |
| β-sitosterol | 0.944 * | 0.915 * | 0.960 ** | 0.996 ** | 0.929 * | |
| Fucosterol | 0.939 * | 0.895 * | 0.925 * | 0.819 | ||
| Squalene | 0.932 * | 0.902 * | 0.864 | |||
| FRAP | 0.974 ** | 0.986 ** | ||||
| DPPH | 0.955 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zheng, C.; Huang, F.; Liu, C.; Huang, Y.; Wang, W. Effects of Radio Frequency Pretreatment on Quality of Tree Peony Seed Oils: Process Optimization and Comparison with Microwave and Roasting. Foods 2021, 10, 3062. https://doi.org/10.3390/foods10123062
Wang Z, Zheng C, Huang F, Liu C, Huang Y, Wang W. Effects of Radio Frequency Pretreatment on Quality of Tree Peony Seed Oils: Process Optimization and Comparison with Microwave and Roasting. Foods. 2021; 10(12):3062. https://doi.org/10.3390/foods10123062
Chicago/Turabian StyleWang, Zhi, Chang Zheng, Fenghong Huang, Changsheng Liu, Ying Huang, and Weijun Wang. 2021. "Effects of Radio Frequency Pretreatment on Quality of Tree Peony Seed Oils: Process Optimization and Comparison with Microwave and Roasting" Foods 10, no. 12: 3062. https://doi.org/10.3390/foods10123062
APA StyleWang, Z., Zheng, C., Huang, F., Liu, C., Huang, Y., & Wang, W. (2021). Effects of Radio Frequency Pretreatment on Quality of Tree Peony Seed Oils: Process Optimization and Comparison with Microwave and Roasting. Foods, 10(12), 3062. https://doi.org/10.3390/foods10123062