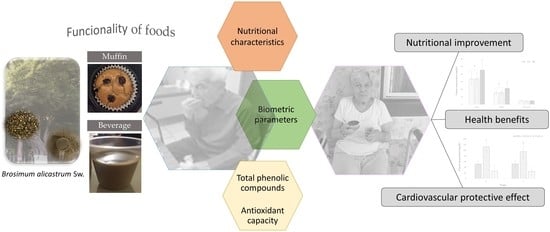
Functionality of Bread and Beverage Added with Brosimum alicastrum Sw. Seed Flour on the Nutritional and Health Status of the Elderly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials for Foods
2.2. Bread and Beverage for Elderly
2.3. Participants of Study
2.4. Anthropometric Measurements and Grip Strength
2.5. Body Composition
2.6. Sarcopenic Status
2.7. Nutritional Status
2.8. Dietary Intake
2.9. Biometric Parameters
2.9.1. Samples
2.9.2. Blood Chemistry Tests
2.9.3. Hematic Biometry
2.9.4. General Urine Test
2.10. Phytochemical Characteristics
2.10.1. Total Phenolic Compounds
2.10.2. Antioxidant Capacity
2.11. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics, Safety, and Acceptance of Foods for Elderly
3.2. Functionality of Foods Designed for the Elderly
3.2.1. Nutritional Indicators of Elderly
3.2.2. Biometric Parameters of Elderly
3.2.3. Phytochemical Indicators in Plasma of Elderly
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OMS. Informe Mundial Sobre el Envejecimiento y la Salud; Ediciones de la OMS: Washington, DC, USA, 2015; ISBN 9789240694873. [Google Scholar]
- Baugreet, S.; Hamill, R.M.; Kerry, J.P.; McCarthy, S.N. Mitigating Nutrition and Health Deficiencies in Older Adults: A Role for Food Innovation? J. Food Sci. 2017, 82, 848–855. [Google Scholar] [CrossRef] [Green Version]
- Triana, F.; Martín, P. Impacto del progresivo envejecimiento de la población en el soporte nutritivo del paciente. Particularidades del anciano en diversas patologías agudas. Análisis en sus distintas vertientes: Hospital, residencias y domicilio. Nutr. Hosp. 2013, 6, 49–59. [Google Scholar]
- Mareschal, J.; Genton, L.; Collet, T.H.; Graf, C. Nutritional intervention to prevent the functional decline in community-dwelling older adults: A systematic review. Nutrients 2020, 12, 2820. [Google Scholar] [CrossRef]
- Burgos, R. Sarcopenia en ancianos. Endocrinol. Nutr. 2006, 53, 335–344. [Google Scholar] [CrossRef]
- Bloom, I.; Shand, C.; Cooper, C.; Robinson, S.; Baird, J. Diet quality and sarcopenia in older adults: A systematic review. Nutrients 2018, 10, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motohashi, N.; Gallagher, R.; Anuradha, V.; Gollapud, R. Functional foods and their importance in geriatric nutrition. J. Clin. Nutr. Metab. 2017, 1, 1–5. [Google Scholar]
- Larqué-Saavedra, A. El sector forestal en apoyo a la cruzada contra el hambre. For. XXI 2014, 17, 11–12. [Google Scholar]
- USDA. Full Report (All Nutrients) 12005 Seeds, Breadnut Tree Seeds, Dried; USDA: Washington, DC, USA, 2016.
- Carter, C.T. Chemical and Functional Properties of Brosimum Alicastrum Seed Powder (Maya Nut, Ramón Nut); Clemson University: Clemson, SC, USA, 2015. [Google Scholar]
- Subiria-Cueto, R.; Larqué-Saavedra, A.; Reyes-Vega, M.L.; de la Rosa, L.A.; Santana-Contreras, L.E.; Gaytán-Martínez, M.; Vázquez-Flores, A.A.; Rodrigo-García, J.; Corral-Avitia, A.Y.; Núñez-Gastélum, J.A.; et al. Brosimum alicastrum Sw. (Ramón): An alternative to improve the nutritional properties and functional potential of the wheat flour tortilla. Foods 2019, 8, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secretaría de Economía. NMX-F-608-NORMEX-2002. Alimentos-Determinación de Proteína en Alimentos. Método de Ensayo (Prueba); Secretaría de Economía, Norma Oficial Mexicana: Ciudad de México, Mexico, 2011; p. 1.
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Horwitz, W., Ed.; AOAC International: Washington, DC, USA, 2000. [Google Scholar]
- Secretaría de Salud. NOM-184-SSA1-2002. Productos y Servicios. Leche, Fórmula Láctea y Producto Lácteo Combinado. Especificaciones Sanitarias; Secretaría de Salud, Norma Oficial Mexicana: Ciudad de México, Mexico, 2002; pp. 1–50.
- Secretaría de Salud. NOM-247-SSA1-2008. Productos y Servicios. Cereales y sus Productos. Cereales, Harinas de Cereales, Sémolas o Semolinas. Alimentos a Base de: Cereales, Semillas Comestibles, de Harinas, Sémolas o Semolinas o sus Mezclas; Secretaría de Salud, Norma Oficial Mexicana: Ciudad de México, Mexico, 2008; pp. 1–117.
- Lawless, H.; Heymann, H. Sensory Evaluation of Food Principles and Practices, 2nd ed.; Springer: New York, NY, USA, 2010; ISBN 978-1-4419-6487-8. [Google Scholar]
- Pfeiffer, E. Short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef]
- Yesavage, J.; Brink, T.; Rose, T.; Lum, O.; Huang, V.; Adey, M. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1983, 17, 37–49. [Google Scholar] [CrossRef]
- Golberg, D.; Bridges, K.; Duncan-Jones, P.; Grayson, D. Detecting anxiety and depression in general medical settings. BMJ 1988, 297, 897–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chumlea, W.C.; Roche, A.F.; Mukherjee, D. Nutritional Assessment of the Elderly through Anthropometry; Ross Laboratories: Columbus, OH, USA, 1984. [Google Scholar]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M. Sarcopenia: European consensus on definition and diagnosis Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.; et al. Extended group EWGSOP2 Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.-L. The mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef]
- Porca, C.; Tejera, C.; Bellido, V.; García, J.; Bellido, D. Nuevo enfoque en la valoración de la ingesta dietética Key words. Nutr. Clin. Med. 2016, 95, 95–107. [Google Scholar] [CrossRef]
- Henry, J.B.; Davey, F.R.; Herman, C.J.; McPherson, R.A.; Pincus, M.R.; Thretle, G.A.; Woods, G.L. El Laboratorio en el Diagnóstico Clínico; Marban Libros, S.L.: Madrid, Spain, 2007; ISBN 9788471015495. [Google Scholar]
- Alvarez-Parrilla, E.; De La Rosa, L.A.; Amarowicz, R.; Shahidi, F. Antioxidant activity of fresh and processed Jalapeño and Serrano peppers. J. Agric. Food Chem. 2011, 59, 163–173. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, L.A.; Alvarez-Parrilla, E.; Shahidi, F. Phenolic compounds and antioxidant activity of kernels and shells of Mexican pecan (Carya illinoinensis). J. Agric. Food Chem. 2011, 59, 152–162. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; de la Rosa, L.A.; Legarreta, P.; Saenz, L.; Rodrigo-García, J.; Gonzalez-Aguilar, G.A. Daily consumption of apple, pear and orange juice differently affects plasma lipids and antioxidant capacity of smoking and non-smoking adults. Int. J. Food Sci. Nutr. 2010, 61, 369–380. [Google Scholar] [CrossRef]
- USDA. National Nutrient Database for Standard Reference; Agricultural Research Service: Washington, DC, USA, 2019.
- Martínez-Ruiz, N.; Javier-Torres, L.; del Hierro-Ochoa, J.; Larqué-Saavedra, A. Bebida adicionada con Brosimum alicastrum Sw.: Una alternativa para requerimientos dietarios especiales. Rev. Salud Pública Nutr. 2019, 18, 1–10. [Google Scholar] [CrossRef]
- Wood, R.J.; Suter, P.M.; Russell, R.M. Mineral requirements of elderly people. Am. J. Clin. Nutr. 1995, 62, 493–505. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guideline: Potassium Intake for Adults and Children; WHO Press: Geneva, Switzerland, 2012; ISBN 978 92 4 150482 9. [Google Scholar]
- WHO. Guideline: Sodium Intake for Adults and Children; WHO Press: Geneva, Switzerland, 2012; ISBN 978 92 4 150483 6. [Google Scholar]
- Bullermann, L.B.; Bianchini, A. The microbiology of cereals and cereal products. Food Qual. Saf. 2011, 1–3. Available online: https://www.foodqualityandsafety.com/article/the-microbiology-of-cereals-and-cereal-products/ (accessed on 12 May 2021).
- Rodríguez, M.; Santamaría, A.; Rivero, M. Alimentos funcionales, complementos alimenticios y productos dietéticos para la edad avanzada. Ambito Farm. 2001, 20, 102–110. [Google Scholar]
- Ozer, H.K. Phenolic compositions and antioxidant activities of Maya nut (Brosimum alicastrum): Comparison with commercial nuts. Int. J. Food Prop. 2017, 20, 2772–2781. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Lu, F.; Li, Z.; Zhao, L.; Han, C. Recent developments in gluten-free bread baking approaches: A review. Food Sci. Technol. 2017, 37, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bourekoua, H.; Różyło, R.; Gawlik-Dziki, U.; Benatallah, L.; Zidoune, M.N.; Dziki, D. Pomegranate seed powder as a functional component of gluten-free bread (Physical, sensorial and antioxidant evaluation). Int. J. Food Sci. Technol. 2018, 53, 1906–1913. [Google Scholar] [CrossRef]
- Dahl, W. Modified Texture Food Production: A Manual for Patient Care Facilities, 2nd ed.; Dietitians of Canada: Toronto, ON, Canada, 2008. [Google Scholar]
- Bourges, H.; Casanueva, E.; Rosado, J. Recomendaciones de Ingestión de Nutrimentos para Población Mexicana. Bases Fisiológicas. Tomo 2. Energía, Proteínas, Lípidos, Hidratos de Carbono y Fibra; Editorial Médica Panamericana: Ciudad de México, Mexico, 2008; ISBN 9789687988597. [Google Scholar]
- Bourges, H.; Casanueva, E.; Rosado, J. Recomendaciones de Ingestión de Nutrimentos para Población Mexicana. Bases Fisiológicas. Tomo 1. Vitaminas y Nutrimentos Inorgánicos; Editorial Médica Panamericana: Ciudad de México, Mexico, 2005; ISBN 9789687988580. [Google Scholar]
- Nyberg, M.; Olsson, V.; Örtman, G.; Pajalic, Z.; Andersson, H.S.; Blücher, A.; Lindborg, A.L.; Wendin, K.; Westergren, A. The meal as a performance: Food and meal practices beyond health and nutrition. Ageing Soc. 2018, 38, 83–107. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Tadeo, A.; Wall-Medrano, A.; Gaytan-Vidana, M.E.; Campos, A.; Ornelas-Contreras, M.; Novelo-Huerta, H.I. Malnutrition risk factors among the elderly from the US-Mexico Border: The “one thousand” study. J. Nutr. Health Aging 2012, 16, 426–431. [Google Scholar] [CrossRef] [PubMed]
- del Velázquez Alva, M.C.; Irigoyen Camacho, M.E.; Delgadillo Velázquez, J.; Lazarevich, I. Relación entre sarcopenia, desnutrición, movilidad física y actividades básicas de la vida diaria en un grupo de ancianas de la ciudad de México. Nutr. Hosp. 2013, 28, 514–521. [Google Scholar] [CrossRef]
- Cheng, H.; Kong, J.; Underwood, C.; Petocz, P.; Hirani, V.; Dawson, B.; O’Leary, F. Systematic review and meta-analysis of the effect of protein and amino acid supplements in older adults with acute or chronic conditions. Br. J. Nutr. 2018, 119, 527–542. [Google Scholar] [CrossRef]
- Alemán-Mateo, H.; Carreón, V.R.; Macías, L.; Astiazaran-García, H.; Gallegos-Aguilar, A.C.; Enríquez, J.R.R. Nutrient-rich dairy proteins improve appendicular skeletal muscle mass and physical performance, and attenuate the loss of muscle strength in older men and women subjects: A single-blind randomized clinical trial. Clin. Interv. Aging 2014, 9, 1517–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rondanelli, M.; Klersy, C.; Terracol, G.; Talluri, J.; Maugeri, R.; Guido, D.; Faliva, M.A.; Solerte, B.S.; Fioravanti, M.; Lukaski, H.; et al. Whey protein, amino acids, and Vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am. J. Clin. Nutr. 2016, 103, 830–840. [Google Scholar] [CrossRef]
- Rus, G.E.; Porter, J.; Brunton, A.; Crocker, M.; Kotsimbos, Z.; Percic, J.; Polzella, L.; Willet, N.; Huggins, C.E. Nutrition interventions implemented in hospital to lower risk of sarcopenia in older adults: A systematic review of randomised controlled trials. Nutr. Diet. 2020, 77, 90–102. [Google Scholar] [CrossRef]
- Rondanelli, M.; Nichetti, M.; Peroni, G.; Faliva, M.A.; Naso, M.; Gasparri, C.; Perna, S.; Oberto, L.; Di Paolo, E.; Riva, A.; et al. Where to find leucine in food and how to feed elderly with sarcopenia in order to counteract loss of muscle mass: Practical advice. Front. Nutr. 2021, 7, 383. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz-Góngora, V.; Martínez-Tapia, B.; Cuevas-Nasu, L.; Flores-Aldana, M.; Shamah-Levy, T. Dietary intake and adequacy of energy and nutrients in Mexican older adults: Results from two National Health and Nutrition Surveys. Salud Publica Mex. 2017, 59, 285–298. [Google Scholar] [CrossRef] [Green Version]
- Naseeb, M.A.; Volpe, S.L. Protein and exercise in the prevention of sarcopenia and aging. Nutr. Res. 2017, 40, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Beelen, J.; Laan, A.C.M.; Poon, S.; de Groot, L.C.P.G.M.; Seeman, E.; Wang, X.; Iuliano, S. An even distribution of protein intake daily promotes protein adequacy but does not influence nutritional status in institutionalized elderly. J. Am. Med. Dir. Assoc. 2018, 19, 33–39. [Google Scholar] [CrossRef]
- Beelen, J.; de Roos, N.M.; de Groot, L.C.P.G.M. Protein enrichment of familiar foods as an innovative strategy to increase protein intake in institutionalized elderly. J. Nutr. Health Aging 2017, 21, 173–179. [Google Scholar] [CrossRef]
- Donini, L.M.; Savina, C.; Cannella, C. Nutrition in the elderly: Role of fiber. Arch. Gerontol. Geriatr. 2009, 49, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Rojas, D.; Nilsson, A.; Santoro, A.; Franceschi, C.; Bazzocchi, A.; Battista, G.; de Groot, L.; Feskens, E.; Berendsen, A.; Pietruszka, B.; et al. Dietary fibre may mitigate sarcopenia risk: Findings from the NU-AGE cohort of older European adults. Nutrients 2020, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Assis, B.S.; Jairza, J.M.B.-M.; Lopes, J.A.; Roriz, A.K.C.; Melo, A.L.; Previdell, A.; Aquino, R.D.C.; Ramos, L.B. Micronutrient intake in elderly living in nursing homes. Nutr. Hosp. 2018, 35, 59. [Google Scholar] [CrossRef] [Green Version]
- Alqabbani, H.M.; AlBadr, N.A. Zinc status (intake and level) of healthy elderly individuals in Riyadh and its relationship to physical health and cognitive impairment. Clin. Nutr. Exp. 2020, 29, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Wawer, A.A.; Jennings, A.; Fairweather-Tait, S.J. Iron status in the elderly: A review of recent evidence. Mech. Ageing Dev. 2018, 175, 55–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckett, E.L.; Martin, C.; Boyd, L.; Porter, T.; King, K.; Niblett, S.; Yates, Z.; Veysey, M.; Lucock, M. Reduced plasma homocysteine levels in elderly Australians following mandatory folic acid fortification—A comparison of two cross-sectional cohorts. J. Nutr. Intermed. Metab. 2017, 8, 14–20. [Google Scholar] [CrossRef]
- Félix-Redondo, F.J.; Grau, M.; Fernández-Bergés, D. Cholesterol and cardiovascular disease in the elderly. Facts and gaps. Aging Dis. 2013, 4, 154–169. [Google Scholar]
- Delahoy, P.J.; Magliano, D.J.; Webb, K.; Grobler, M.; Liew, D. The relationship between reduction in low-density lipoprotein cholesterol by statins and reduction in risk of cardiovascular outcomes: An updated meta-analysis. Clin. Ther. 2009, 31, 236–244. [Google Scholar] [CrossRef]
- Vallejo-Vaz, A.J.; Ray, K.K.; Ginsberg, H.N.; Davidson, M.H.; Eckel, R.H.; Lee, L.V.; Bessac, L.; Pordy, R.; Letierce, A.; Cannon, C.P. Associations between lower levels of low-density lipoprotein cholesterol and cardiovascular events in very high-risk patients: Pooled analysis of nine ODYSSEY trials of alirocumab versus control. Atherosclerosis 2019, 288, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Matsui, S.; Kajikawa, M.; Hida, E.; Maruhashi, T.; Iwamoto, Y.; Iwamoto, A.; Oda, N.; Kishimoto, S.; Hidaka, T.; Kihara, Y.; et al. Optimal target level of low-density lipoprotein cholesterol for vascular function in Statin Naïve individuals. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, S.; Bruckert, E.; Gallo, A.; Plat, J. The position of functional foods and supplements with a serum LDL-C lowering effect in the spectrum ranging from universal to care-related CVD risk management. Atherosclerosis 2020, 311, 116–123. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizarro, S.; Ronco, A.M.; Gotteland, M. β-glucanos: ¿Qué tipos existen y cuáles son sus beneficios en la salud? Rev. Chil. Nutr. 2014, 41, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Hosten, A. BUN and Creatinine. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walher, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths-Heinemann: Boston, MA, USA, 1990; p. 1118. ISBN 9780409900774. [Google Scholar]
- Carbone, J.W.; Pasiakos, S.M. Dietary protein and muscle mass: Translating science to application and health benefit. Nutrients 2019, 11, 1136. [Google Scholar] [CrossRef] [Green Version]
- Lutz, M.; Petzold, G.; Albala, C. Considerations for the development of innovative foods to improve nutrition in older adults. Nutrients 2019, 11, 1275. [Google Scholar] [CrossRef] [Green Version]
- Stout, J. Sarcopenia. Nutr. Hosp. 2011, 4, 7–8. [Google Scholar]
- Michelfelder, A.J. Soy: A complete source of protein. Am. Fam. Physician 2009, 79, 43–47. [Google Scholar] [PubMed]
- Peña-Ordóñez, G.G.; Bustamante-Montes, L.P.; Ramírez-Duran, N.; Halley-Castillo, E.; García-Cáceres, L. Evaluación de la ingesta proteica y la actividad física asociadas con la sarcopenia del adulto mayor. Rev. Esp. Nutr. Hum. Diet. 2015, 20, 16. [Google Scholar] [CrossRef] [Green Version]
- Goñi, I.; Hern, A. Antioxidants. Contribution of macromolecular Mediterranean population. Nutrients 2019, 11, 2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [Green Version]




| RSF * | Muffin | Beverage | |
|---|---|---|---|
| Energy (kcal/100 g) | 336 | 353 | 54 |
| Moisture (%) | 13.3 ± 0.14 | 36.3 ± 0.22 | 81.8 ± 0.06 |
| Protein (%) | 11.5 ± 0.39 | 18.1 ± 0.12 | 5.5 ± 0.07 |
| Fat (%) | 0.6 ± 0.00 | 25.3 ± 0.24 | 0.1 ± 0.00 |
| Ashes (%) | 3.4 ± 0.11 | 1.8 ± 0.05 | 0.7 ± 0.01 |
| Total carbohydrates (%) | 71.2 ± 0.56 | 18.5 ± 0.23 | 11.9 ± 0.01 |
| Total sugars (%) | NS | 6.2 ± 0.20 | 3.3 ± 0.01 |
| Dietary fiber (%) | 13.0 ± 0.21 | 5.1 ± 0.20 | 4.3 ± 0.10 |
| pH | 5.5 ± 0.01 | 8.0 ± 0.11 | 6.3 ± 0.00 |
| Activity water (Aw) | 0.30 ± 0.02 | 0.95 ± 0.00 | 96.0 ± 0.04 |
| Cu (mg/100 g) | 0.5 ± 0.10 | 0.3 ± 0.00 | 0.2 ± 0.01 |
| Zn (mg/100g) | 1.0 ± 0.10 | 1.7 ± 0.02 | 0.2 ± 0.00 |
| Fe (mg/100 g) | 4.0 ± 0.70 | 5.5 ± 0.01 | 0.7 ± 0.00 |
| K (mg/100 g) | 1256.0 ± 12.00 | 323.7 ± 5.82 | 178.9 ± 4.51 |
| Na (mg/100 g) | 47.0 ± 0.10 | 245.2 ± 3.96 | 78.7 ± 4.77 |
| TPC (mg GAE/100 g extract) † | 89.8 ± 3.2 | 268.7 ± 11.8 | 149.3 ± 6.8 |
| AC (FRAP) (μmol TE/100 g extract) † | 209.6 ± 8.1 | 637.7 ± 36.6 | 268.3 ± 11.9 |
| AC (ABTS) (μmol TE/100 g extract) † | 371.5 ± 23.2 | 1553.4 ± 75.3 | 691.2 ± 28.9 |
| Men | Women | p | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Height (cm) | 162.7 | 5.9 | 149.7 | 2.6 | 0.000 |
| Weight (kg) | 59.7 | 10.8 | 55.4 | 10.9 | 0.171 |
| TBW (L) | 29.5 | 3.9 | 25.4 | 2.7 | 0.013 |
| FFM (kg) | 40.3 | 5.4 | 34.7 | 3.7 | 0.013 |
| FM (kg) | 18.5 | 6.4 | 17.6 | 5.3 | 0.728 |
| BMI (kg/m2) | 21.4 | 3.7 | 23.4 | 1.8 | 0.244 |
| Parameters | B | C | B–C p | I | C–I p |
|---|---|---|---|---|---|
| Body weight (kg) | 56.3 ± 9.6 | 55.9 ± 9.5 | 0.59 | 57.0 ± 9.3 | <0.01 |
| Arm circumference (cm) | 29.0 ± 12.2 | 26.2 ± 3.1 | 0.33 | 26.0 ± 3.2 | 0.50 |
| Calf circumference (cm) | 30.7 ± 2.9 | 30.6 ± 2.9 | 0.45 | 31.0 ± 3.0 | 0.14 |
| TBW (L) | 27.9 ± 4.0 | 27.0 ± 4.9 | 0.05 | 27.1 ± 5.0 | 0.88 |
| FFM (kg) | 38.1 ± 5.5 | 36.3 ± 7.2 | <0.01 | 37.0 ± 6.8 | 0.37 |
| FM (kg) | 18.1 ± 5.9 | 18.6 ± 5.7 | 0.65 | 20.1 ± 6.3 | 0.11 |
| BMI (kg/m2) | 22.1 ± 3.3 | 22.6 ± 3.6 | 0.23 | 22.8 ± 3.4 | 0.85 |
| Grip strenght (kg) | 15.8 ± 6.7 | 16.3 ± 7.3 | 0.29 | 16.5 ± 6.2 | 0.80 |
| Cognitive assesment | 2.5 ± 1.9 | 2.7 ± 2.3 | 0.63 | 2.3 ± 2.6 | 0.35 |
| MNA | 24.2 ± 4.0 | 24.8 ±3.8 | 0.19 | 24.3 ± 3.4 | 0.44 |
| Stage | |||
|---|---|---|---|
| C | I | p | |
| Energy (kcal/d) | 1547 ± 224 | 1697 ± 287 | 0.24 |
| Macronutrients | |||
| Protein (g/d) | 63.8 ± 12.6 | 82.4 ± 14.5 | <0.01 |
| Total fat (g/d) | 48.0 ± 10.0 | 54.3 ± 14.4 | 0.30 |
| Satured fatty acids (g/d) | 11.0 ± 1.0 | 11.8 ± 2.1 | 0.26 |
| Monounsatured fatty acids (g/d) | 15.4 ± 3.0 | 10.7 ± 3.2 | <0.01 |
| Polyunsaturated fatty acids (g/d) | 8.9 ± 2.1 | 6.3 ± 1.8 | <0.01 |
| Omega-6 fatty acids (g/d) | 5.3 ± 1.6 | 3.8 ± 1.2 | 0.01 |
| Omega-3 fatty acids (g) | 0.4 ± 0.2 | 0.8 ± 0.2 | <0.01 |
| Carbohydrates (g/d) | 222.4 ± 28.8 | 215.8 ± 33.4 | 0.66 |
| Total fiber (g/d) | 17.6 ± 9.1 | 31.0 ± 5.9 | <0.01 |
| Total cholesterol (mg/d) | 320.7 ± 58.5 | 254.7 ± 85.9 | <0.01 |
| Minerals | |||
| Calcium (mg/d) | 726.5 ± 178.0 | 684.3 ± 126.0 | 0.39 |
| Iron (mg/d) | 9.9 ± 2.5 | 14.3 ± 2.8 | <0.01 |
| Potassium (mg/d) | 1351.9 ± 467.5 | 1443.1 ± 290.1 | 0.34 |
| Sodium (mg/d) | 2001.3 ± 545.8 | 1681.8 ± 588.9 | 0.03 |
| Zinc (mg/d) | 4.6 ± 1.2 | 6.4 ± 2.4 | <0.01 |
| Vitamins | |||
| Vitamin C (mg/d) | 60.7 ± 15.1 | 44.0 ± 13.6 | <0.01 |
| Folate (µg/d) | 425.3 ± 204.0 | 508.7 ± 142.5 | 0.03 |
| Vitamin A (µg RAE/d) | 273.8 ± 154.8 | 366.4 ± 295.6 | 0.25 |
| Vitamin E (mg/d) | 4.4 ± 3.1 | 2.5 ± 1.6 | <0.01 |
| Parameter | B | C | I | RV |
|---|---|---|---|---|
| Blood Chemestry | ||||
| Glucose (mg/dL) | 92.0 ± 14.4 a | 79.9 ± 7.6 bB | 87.2 ± 9.6 A | 60.0–100.0 |
| Triglycerides (mg/dL) | 119.8 ± 47.4 | 122.3 ± 43.2 | 130.7 ± 59.4 | 45.0–191.0 |
| Total cholesterol (mg/dL) | 187.0 ± 21.1 | 191.0 ± 33.6 | 176.5 ± 34.2 | 120.0–200.0 |
| HDL-C (mg/dL) | 49.9 ± 11.3 | 51.8 ± 11.3 | 52.3 ± 12.5 | 36.0–76.0 |
| LDL-C (mg/dL) | 105.5 ± 17.8 | 112.5 ± 26.0 A | 95.8 ± 30.1 B | 80.0–150.0 |
| VLDL-C (mg/dL) | 28.5 ± 21.4 | 26.7 ± 13.6 | 26.2 ± 11.9 | 0.0–45.0 |
| Total protein (g/dL) | 7.2 ± 0.5 | 7.4 ± 0.6 A | 6.9 ± 0.6 B | 6.0–8.5 |
| Albumin (g/dL) | 4.4 ± 0.4 a | 4.1 ± 0.3 b | 4.2 ± 0.3 | 3.5–5.2 |
| CRP (mg/dL) | 0.31 ± 0.3 | 0.35 ± 0.3 | 0.40 ± 0.5 | 0.0–5.0 |
| Creatinine (mg/dL) | 0.80 ± 0.1 | 0.74 ± 0.1 | 0.83 ± 0.2 | 0.5–1.5 |
| Urea (mg/dL) | 33.4 ± 8.0 | 34.1 ± 7.6 B | 45.4 ± 11.3 A | 13.0–60.0 |
| BUN (mg/dL) | 15.6 ± 3.7 | 15.9 ± 3.4 B | 21.2 ± 5.3 A | 6.0–28.0 |
| Uric acid (mg/dL) | 4.8 ± 1.0 | 4.4 ± 0.9 | 4.9 ± 1.1 | 2.7–7.2 |
| Hematic biometric | ||||
| Erytrocytes (×106/μL) | 4.5 ± 0.4 | 4.7 ± 1.0 | 4.3 ± 0.5 | 4.1–5.1 |
| Hemoglobin (g/dL) | 14.1 ± 1.3 | 13.9 ± 1.5 | 13.5 ± 1.5 | 12.3–15.3 |
| Hematocrit (%) | 43.1 ± 3.7 | 42.7 ± 4.8 | 41.2 ± 4.8 | 36.0–45.0 |
| MCV (fL) | 95.3 ± 4.3 | 95.4 ± 3.8 | 95.4 ± 3.9 | 80.0–100.0 |
| MCH (pg) | 31.2 ± 1.8 | 31.0 ± 1.5 | 32.5 ± 0.8 | 27.5–32.2 |
| MCHC (g/dL) | 32.7 ± 0.9 † | 32.5 ± 0.8 † | 32.7 ± 0.9 † | 33.4–35.5 |
| RDW (%) | 14.1 ± 0.7 | 13.9 ± 0.8 | 13.9 ± 0.9 | 11.5–16.0 |
| HD (fL) | 48.3 ± 3.3 | 47.4 ± 2.9 | 47.1 ± 3.1 | 0.0–99.9 |
| Lukocytes (×103/μL) | 6.4 ± 1.3 | 6.9 ± 2.3 | 6.5 ± 1.7 | 4.5–11.0 |
| Neutrophils (%) | 60.1 ± 9.9 | 61.6 ± 8.2 | 60.8 ± 7.8 | 37.0–72.0 |
| Lymphocytes (%) | 27.3 ± 8.6 | 25.9 ± 7.5 | 26.7 ± 6.9 | 20.0–50.0 |
| Monocytes (%) | 8.6 ± 3.1 | 8.9 ± 2.7 | 8.9 ± 2.9 | 0.0–14.0 |
| Eosinophils (%) | 3.6 ± 3.9 | 3.0 ± 2.2 | 3.1 ± 2.0 | 0.0–6.0 |
| Basophils (%) | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.0–1.0 |
| Platelets (×103/μL) | 224.6 ± 68.5 | 236.3 ± 80.0 | 233.9 ± 72.4 | 140.0–450.0 |
| Platelet volume (fL) | 10.5 ± 1.0 † | 11.2 ± 1.3 † | 10.7 ± 1.1 † | 7.4–10.4 |
| General urine analisys | ||||
| Colour ** | Yellow | Yellow | Yellow | |
| Appearance ** | 40% clear | 50% clear | 61% clear | |
| Density | 1.015 ± 0.007 | 1.015 ± 0.006 | 1.017 ± 0.009 | 1.002–1.030 |
| pH | 6.0 ± 1.2 | 6.3 ± 0.8 | 5.8 ± 0.9 | 4.5–8.0 |
| Glucose ** | 100% negative | 100% negative | 100% negative | Negative |
| Ketone bodies ** | 100% negative | 100% negative | 100% negative | Negative |
| Urobilinogen ** | 95% normal | 100% normal | 100% normal | 0.2 EU/dL |
| Bilirubin ** | 95% negative | 95% negative | 100% negative | Negative |
| Leucocyte esterase ** | 65.2% negative | 69.6% negative | 69.6% negative | Negative |
| Nitrites ** | 87.0% negative | 91.3% negative | 82.6% negative | Negative |
| Proteins ** | 87.0% negative | 82.6% negative | 82.6% negative | Negative |
| Hemoglobin ** | 73.9% negative | 69.6% negative | 69.6% negative | Negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Tadeo, A.; del Hierro-Ochoa, J.C.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; de la Rosa, L.A.; Alvarez-Parrilla, E.; López-Díaz, J.A.; Vidaña-Gaytán, M.E.; González-Valles, M.N.; Larqué-Saavedra, A.; et al. Functionality of Bread and Beverage Added with Brosimum alicastrum Sw. Seed Flour on the Nutritional and Health Status of the Elderly. Foods 2021, 10, 1764. https://doi.org/10.3390/foods10081764
Rodríguez-Tadeo A, del Hierro-Ochoa JC, Moreno-Escamilla JO, Rodrigo-García J, de la Rosa LA, Alvarez-Parrilla E, López-Díaz JA, Vidaña-Gaytán ME, González-Valles MN, Larqué-Saavedra A, et al. Functionality of Bread and Beverage Added with Brosimum alicastrum Sw. Seed Flour on the Nutritional and Health Status of the Elderly. Foods. 2021; 10(8):1764. https://doi.org/10.3390/foods10081764
Chicago/Turabian StyleRodríguez-Tadeo, Alejandra, Julio C. del Hierro-Ochoa, Jesús O. Moreno-Escamilla, Joaquín Rodrigo-García, Laura A. de la Rosa, Emilio Alvarez-Parrilla, José A. López-Díaz, María E. Vidaña-Gaytán, María N. González-Valles, Alfonso Larqué-Saavedra, and et al. 2021. "Functionality of Bread and Beverage Added with Brosimum alicastrum Sw. Seed Flour on the Nutritional and Health Status of the Elderly" Foods 10, no. 8: 1764. https://doi.org/10.3390/foods10081764
APA StyleRodríguez-Tadeo, A., del Hierro-Ochoa, J. C., Moreno-Escamilla, J. O., Rodrigo-García, J., de la Rosa, L. A., Alvarez-Parrilla, E., López-Díaz, J. A., Vidaña-Gaytán, M. E., González-Valles, M. N., Larqué-Saavedra, A., & Martínez-Ruiz, N. d. R. (2021). Functionality of Bread and Beverage Added with Brosimum alicastrum Sw. Seed Flour on the Nutritional and Health Status of the Elderly. Foods, 10(8), 1764. https://doi.org/10.3390/foods10081764