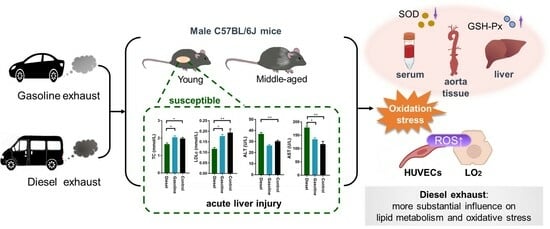
Lipid Dysregulation Induced by Gasoline and Diesel Exhaust Exposure and the Interaction with Age
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Exposure
2.1.1. Experimental Animals
2.1.2. Exposure Process in Mice
2.1.3. Biochemical Analysis of Serum
2.1.4. Determination of Oxidative Stress Indicators
2.2. In Vitro Experiment
2.2.1. PM2.5 Collection
2.2.2. Cell Culture
2.2.3. CCK-8 Assay for Cell Viability
2.2.4. Reactive Oxygen Species (ROS) Levels
2.3. Statistical Analysis
3. Results
3.1. Effect on Biochemical Parameters in Mice
3.2. Effect on Oxidative Stress in Mice
3.2.1. Serum
3.2.2. Aorta
3.2.3. Liver
3.3. Effect on Cell Viability of HUVECs and LO2
3.4. Effect on ROS Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | black carbon; |
| EC | elemental carbon; |
| CO | carbon monoxide; |
| HC | hydrocarbons; |
| NOx | nitrogen oxides; |
| NO2 | nitrogen dioxide; |
| PM | Particulate Matter; |
| OR | Odds Ratio; |
| DEP | diesel engine exhaust particles; |
| ROS | reactive oxygen species; |
| HUVECs | human umbilical vein endothelial cells; |
| LO2 | human normal liver cells; |
| GE | gasoline exhaust; |
| DE | diesel exhaust; |
| NEDC | New European Driving Cycle; |
| SMPS | Scanning Mobility Particle Sizer; |
| APS | Aerodynamic Panicle Sizer; |
| TC | Total Cholesterol; |
| TG | Total Triglycerides; |
| HDLc | High-Density Lipoprotein Cholesterol; |
| LDLc | Low-Density Lipoprotein Cholesterol; |
| ALP | alkaline phosphatase; |
| ALT | alanine aminotransferase; |
| AST | aspartate aminotransferase; |
| SOD | Superoxide Dismutase; |
| MDA | Malondialdehyde; |
| GSH-Px | Glutathione Peroxidase; |
| ELISA | enzyme-linked immunosorbent assay; |
| CCK-8 | Cell Counting Kit-8; |
| OD | optical density. |
References
- Wei, P.; Brimblecombe, P.; Yang, F.H.; Anand, A.; Xing, Y.; Sun, L.; Sun, Y.X.; Chu, M.Y.; Ning, Z. Determination of local traffic emission and non-local background source contribution to on-road air pollution using fixed-route mobile air sensor network. Environ. Pollut. 2021, 290, 118055. [Google Scholar] [CrossRef]
- Tolis, E.I.; Karanotas, T.; Svolakis, G.; Panaras, G.; Bartzis, J.G. Air quality in cabin environment of different passenger cars: Effect of car usage, fuel type and ventilation/infiltration conditions. Environ. Sci. Pollut. Res. 2021, 28, 51232–51241. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, Z.; Xu, H.; Du, K. Emission factors of air pollutants from CNG-gasoline bi-fuel vehicles: Part I. Black carbon. Sci. Total Environ. 2016, 572, 1161–1165. [Google Scholar] [CrossRef]
- Malla, S. Assessment of mobility and its impact on energy use and air pollution in Nepal. Energy 2014, 69, 485–496. [Google Scholar] [CrossRef]
- Li, T.; Yang, H.-L.; Xu, L.-T.; Zhou, Y.-T.; Min, Y.-J.; Yan, S.-C.; Zhang, Y.-H.; Wang, X.-M. Comprehensive treatment strategy for diesel truck exhaust. Environ. Sci. Pollut. Res. 2023, 30, 54324–54332. [Google Scholar] [CrossRef]
- Lund, M.T.; Berntsen, T.K.; Heyes, C.; Klimont, Z.; Samset, B.H. Global and regional climate impacts of black carbon and co-emitted species from the on-road diesel sector. Atmos. Environ. 2014, 98, 50–58. [Google Scholar] [CrossRef]
- Breuer, J.L.; Samsun, R.C.; Peters, R.; Stolten, D. The impact of diesel vehicles on NOx and PM10 emissions from road transport in urban morphological zones: A case study in North Rhine-Westphalia, Germany. Sci. Total Environ. 2020, 727, 138583. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, X.; Meng, T.; Wang, H.; Bin, P.; Shen, M.; Chen, W.; Yu, S.; Leng, S.; Zheng, Y. Exposure characterization and estimation of benchmark dose for cancer biomarkers in an occupational cohort of diesel engine testers. J. Expo. Sci. Env. Epidemiol. 2018, 28, 579–588. [Google Scholar] [CrossRef]
- Pan, W.C.; Yeh, S.Y.; Wu, C.D.; Huang, Y.T.; Chen, Y.C.; Chen, C.J.; Yang, H.I. Association Between Traffic Count and Cardiovascular Mortality: A Prospective Cohort Study in Taiwan. J. Epidemiol. 2021, 31, 343–349. [Google Scholar] [CrossRef]
- Habert, C.; Garnier, R. Health effects of diesel exhaust: A state of the art. Rev. Des Mal. Respir. 2015, 32, 138–154. [Google Scholar] [CrossRef]
- Parent, M.E.; Rousseau, M.C.; Boffetta, P.; Cohen, A.; Siemiatycki, J. Exposure to diesel and gasoline engine emissions and the risk of lung cancer. Am. J. Epidemiol. 2007, 165, 53–62. [Google Scholar] [CrossRef]
- Andersen, M.H.G.; Frederiksen, M.; Saber, A.T.; Wils, R.S.; Fonseca, A.S.; Koponen, I.K.; Johannesson, S.; Roursgaard, M.; Loft, S.; Møller, P.; et al. Health effects of exposure to diesel exhaust in diesel-powered trains. Part Fibre Toxicol 2019, 16, 21. [Google Scholar] [CrossRef]
- Vesterdal, L.K.; Danielsen, P.H.; Folkmann, J.K.; Jespersen, L.F.; Aguilar-Pelaez, K.; Roursgaard, M.; Loft, S.; Moller, P. Accumulation of lipids and oxidatively damaged DNA in hepatocytes exposed to particles. Toxicol. Appl. Pharmacol. 2014, 274, 350–360. [Google Scholar] [CrossRef]
- Tseng, C.Y.; Wang, J.S.; Chang, Y.J.; Chang, J.F.; Chao, M.W. Exposure to High-Dose Diesel Exhaust Particles Induces Intracellular Oxidative Stress and Causes Endothelial Apoptosis in Cultured In Vitro Capillary Tube Cells. Cardiovasc. Toxicol. 2015, 15, 345–354. [Google Scholar] [CrossRef]
- Tomaru, M.; Takano, H.; Inoue, K.I.; Yanagisawa, R.; Osakabe, N.; Yasuda, A.; Shimada, A.; Kato, Y.; Uematsu, H. Pulmonary exposure to diesel exhaust particles enhances fatty change of the liver in obese diabetic mice. Int. J. Mol. Med. 2007, 19, 17–22. [Google Scholar] [CrossRef]
- Miller, M.R.; Shaw, C.A.; Langrish, J.P. From particles to patients: Oxidative stress and the cardiovascular effects of air pollution. Future Cardiol. 2012, 8, 577–602. [Google Scholar] [CrossRef]
- Durga, M.; Nathiya, S.; Rajasekar, A.; Devasena, T. Effects of ultrafine petrol exhaust particles on cytotoxicity, oxidative stress generation, DNA damage and inflammation in human A549 lung cells and murine RAW 264.7 macrophages. Environ. Toxicol. Pharmacol. 2014, 38, 518–530. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.L.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate Matter Air Pollution and Cardiovascular Disease An Update to the Scientific Statement From the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Delfino, R.J.; Staimer, N.; Vaziri, N.D. Air pollution and circulating biomarkers of oxidative stress. Air Qual. Atmos. Health 2011, 4, 37–52. [Google Scholar] [CrossRef]
- Tseng, C.-Y.; Chang, J.-F.; Wang, J.-S.; Chang, Y.-J.; Gordon, M.K.; Chao, M.-W. Protective Effects of N-Acetyl Cysteine against Diesel Exhaust Particles-Induced Intracellular ROS Generates Pro-Inflammatory Cytokines to Mediate the Vascular Permeability of Capillary-Like Endothelial Tubes. PLoS ONE 2015, 10, e0131911. [Google Scholar] [CrossRef]
- Chao, M.W.; Kozlosky, J.; Po, I.P.; Strickland, P.O.; Svoboda, K.K.H.; Cooper, K.; Laumbach, R.J.; Gordon, M.K. Diesel exhaust particle exposure causes redistribution of endothelial tube VE-cadherin. Toxicology 2011, 279, 73–84. [Google Scholar] [CrossRef]
- Huang, Y.X.; Unger, N.; Harper, K.; Heyes, C. Global Climate and Human Health Effects of the Gasoline and Diesel Vehicle Fleets. GeoHealth 2020, 4, e2019GH000240. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Batterman, S.A. Proximity of schools in Detroit, Michigan to automobile and truck traffic. J. Expo. Sci. Environ. Epidemiol. 2006, 16, 457–470. [Google Scholar] [CrossRef]
- Athyros, V.G.; Doumas, M.; Imprialos, K.P.; Stavropoulos, K.; Georgianou, E.; Katsimardou, A.; Karagiannis, A. Diabetes and lipid metabolism. Hormones 2018, 17, 61–67. [Google Scholar] [CrossRef]
- Noels, H.; Lehrke, M.; Vanholder, R.; Jankowski, J. Lipoproteins and fatty acids in chronic kidney disease: Molecular and metabolic alterations. Nat. Rev. Nephrol. 2021, 17, 528–542. [Google Scholar] [CrossRef]
- Averyanova, I.V. Age-related blood biochemical changes (lipid metabolism) in healthy young and mature men living under the North conditions. Klin. Lab. Diagn. 2021, 66, 728–732. [Google Scholar] [CrossRef]
- Wu, J.; Bu, D.D.; Wang, H.Q.; Shen, D.; Chong, D.Y.; Zhang, T.Y.; Tao, W.W.; Zhao, M.F.; Zhao, Y.; Fang, L.; et al. The rhythmic coupling of Egr-1 and Cidea regulates age-related metabolic dysfunction in the liver of male mice. Nat. Commun. 2023, 14, 1634. [Google Scholar] [CrossRef]
- Gradinaru, D.; Borsa, C.; Ionescu, C.; Prada, G.I. Oxidized LDL and NO synthesis-Biomarkers of endothelial dysfunction and ageing. Mech. Ageing Dev. 2015, 151, 101–113. [Google Scholar] [CrossRef]
- Perng, W.; Watkins, D.J.; Cantoral, A.; Mercado-García, A.; Meeker, J.D.; Téllez-Rojo, M.M.; Peterson, K.E. Exposure to phthalates is associated with lipid profile in peripubertal Mexican youth. Environ. Res. 2017, 154, 311–317. [Google Scholar] [CrossRef]
- Lee, S.W.; Yon, D.K.; James, C.C.; Lee, S.; Koh, H.Y.; Sheen, Y.H.; Oh, J.W.; Han, M.Y.; Sugihara, G. Short-term effects of multiple outdoor environmental factors on risk of asthma exacerbations: Age-stratified time-series analysis. J. Allergy Clin. Immunol. 2019, 144, 1542–1550. [Google Scholar] [CrossRef]
- Wang, N.; Mengersen, K.; Tong, S.; Kimlin, M.; Zhou, M.; Wang, L.; Yin, P.; Xu, Z.; Cheng, J.; Zhang, Y.; et al. Short-term association between ambient air pollution and lung cancer mortality. Environ. Res. 2019, 179, 108748. [Google Scholar] [CrossRef]
- Song, A.; Peng, J.; Guo, J.; Zhang, J.; Tong, H.; Lv, Z.; Yang, N.; Tang, M.; Mao, H. Interannual variations of vehicle emissions and evaluation of emission control policies based on tunnel measurements in a typical megacity in China. Acta Sci. Circumstantiae 2023, 43, 166–178. [Google Scholar]
- Zhang, Y.; Andre, M.; Liu, Y.; Ren, P.; Yang, Z.; Yuan, Y.; Mao, H. Research on vehicle activity characteristies of typical roads in Tianjin. Environ. Pollut. Control 2018, 40, 365–372. [Google Scholar]
- Zhao, J.B.; Mi, X.Y.; Zhao, L.L.; Midgley, A.C.; Tang, H.Y.; Tian, M.Y.; Yan, H.Y.; Wang, K.; Wang, R.; Wan, Y.J.; et al. Validation of PM2.5 model particle through physicochemical evaluation and atherosclerotic plaque formation in ApoE(-/-) mice. Ecotoxicol. Environ. Saf. 2020, 192, 110308. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, Q. Damage effects of dust storm PM2.5 on DNA in alveolar macrophages and lung cells of rats. Food Chem. Toxicol. 2007, 45, 1368–1374. [Google Scholar] [CrossRef]
- Wang, H.T.; Wang, G.S.; Meng, Y.F.; Liu, Y.Q.; Yao, X.Q.; Feng, C.L. Modified Guo-Min decoction ameliorates PM2.5-induced lung injury by inhibition of PI3K-AKT and MAPK signaling pathways. Phytomedicine 2024, 123, 155211. [Google Scholar] [CrossRef]
- Wang, X.F.; Jiang, S.F.; Liu, Y.; Du, X.Y.; Zhang, W.B.; Zhang, J.; Shen, H.Q. Comprehensive pulmonary metabolome responses to intratracheal instillation of airborne fine particulate matter in rats. Sci. Total Environ. 2017, 592, 41–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Hu, Q.; Mao, H.; Wang, T. Nitro substituent caused negative impact on occurrence and development of atherosclerotic plaque by PM2.5-bound polycyclic aromatic compounds. Sci. Total Environ. 2023, 906, 167700. [Google Scholar] [CrossRef]
- González, C.M.; Gómez, C.D.; Rojas, N.Y.; Acevedo, H.; Aristizábal, B.H. Relative impact of on-road vehicular and point-source industrial emissions of air pollutants in a medium-sized Andean city. Atmos. Environ. 2017, 152, 279–289. [Google Scholar] [CrossRef]
- Tong, Z.; Chen, Y.; Malkawi, A.; Adamkiewicz, G.; Spengler, J.D. Quantifying the impact of traffic-related air pollution on the indoor air quality of a naturally ventilated building. Environ. Int. 2016, 89–90, 138–146. [Google Scholar] [CrossRef]
- Aragon, M.J.; Chrobak, I.; Brower, J.; Roldan, L.; Fredenburgh, L.E.; McDonald, J.D.; Campen, M.J. Inflammatory and Vasoactive Effects of Serum Following Inhalation of Varied Complex Mixtures. Cardiovasc. Toxicol. 2016, 16, 163–171. [Google Scholar] [CrossRef]
- Campen, M.; Robertson, S.; Lund, A.; Lucero, J.; McDonald, J. Engine exhaust particulate and gas phase contributions to vascular toxicity. Inhal. Toxicol. 2014, 26, 353–360. [Google Scholar] [CrossRef]
- Schisler, J.C.; Ronnebaum, S.M.; Madden, M.; Channell, M.; Campen, M.; Willis, M.S. Endothelial inflammatory transcriptional responses to an altered plasma exposome following inhalation of diesel emissions. Inhal. Toxicol. 2015, 27, 272–280. [Google Scholar] [CrossRef]
- Vedal, S.; Campen, M.J.; McDonald, J.D.; Larson, T.V.; Sampson, P.D.; Sheppard, L.; Simpson, C.D.; Szpiro, A.A. National Particle Component Toxicity (NPACT) initiative report on cardiovascular effects. Res. Rep. (Health Eff. Inst.) 2013, 178, 5–8. [Google Scholar]
- Elder, A.; Oberdorster, G. Translocation and effects of ultrafine particles outside of the lung. Clin. Occup. Environ. Med. 2006, 5, 785–796. [Google Scholar]
- Reed, M.D.; Gigliotti, A.P.; McDonald, J.D.; Seagrave, J.C.; Seilkop, S.K.; Mauderly, J.L. Health effects of subchronic exposure to environmental levels of diesel exhaust. Inhal. Toxicol. 2004, 16, 177–193. [Google Scholar] [CrossRef]
- Conklin, D.J.; Kong, M.; Committee, H.E.I.H.R. Part 4. Effects of subchronic diesel engine emissions exposure on plasma markers in rodents: Report on 1- and 3-month exposures in the ACES bioassay. Res. Rep. (Health Eff. Inst.) 2012, 166, 189–223. [Google Scholar]
- Sun, J.H.; Peng, S.X.; Li, Z.Y.; Liu, F.F.; Wu, C.X.; Lu, Y.A.; Xiang, H. Association of Short-Term Exposure to PM2.5 with Blood Lipids and the Modification Effects of Insulin Resistance: A Panel Study in Wuhan. Toxics 2022, 10, 663. [Google Scholar] [CrossRef]
- Rastogi, L.; Dash, K.; Sashidhar, R.B. Selective and sensitive detection of cholesterol using intrinsic peroxidase-like activity of biogenic palladium nanoparticles. Curr. Res. Biotechnol. 2021, 3, 42–48. [Google Scholar] [CrossRef]
- Moon, J.-Y.; Moon, M.H.; Kim, K.T.; Jeong, D.H.; Kim, Y.N.; Chung, B.C.; Choi, M.H. Cytochrome P450-mediated metabolic alterations in preeclampsia evaluated by quantitative steroid signatures. J. Steroid Biochem. Mol. Biol. 2014, 139, 182–191. [Google Scholar] [CrossRef]
- Bochem, A.E.; Holleboom, A.G.; Romijn, J.A.; Hoekstra, M.; Dallinga-Thie, G.M.; Motazacker, M.M.; Hovingh, G.K.; Kuivenhoven, J.A.; Stroes, E.S.G. High density lipoprotein as a source of cholesterol for adrenal steroidogenesis: A study in individuals with low plasma HDL-C. J. Lipid Res. 2013, 54, 1698–1704. [Google Scholar] [CrossRef]
- Ding, L.; Yang, L.; Wang, Z.; Huang, W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm. Sin. B 2015, 5, 135–144. [Google Scholar] [CrossRef]
- Luo, W.; Liu, L.; Yang, L.; Dong, Y.; Liu, T.; Wei, X.; Liu, D.; Gu, H.; Kong, J.; Yuan, Z.; et al. The vitamin D receptor regulates miR-140-5p and targets the MAPK pathway in bone development. Metabolism 2018, 85, 139–150. [Google Scholar] [CrossRef]
- Schade, D.S.; Shey, L.; Eaton, R.P. Cholesterol Review: A Metabolically Important Molecule. Endocr. Pract. 2020, 26, 1514–1523. [Google Scholar] [CrossRef]
- Minuk, G.Y. Canadian Association of Gastroenterology Practice Guidelines: Evaluation of abnormal liver enzyme tests. Can. J. Gastroenterol. 1998, 12, 417–421. [Google Scholar] [CrossRef]
- Dai, S.; Wang, Z.; Yang, Y.; Du, P.; Li, X. PM2.5 induced weight loss of mice through altering the intestinal microenvironment: Mucus barrier, gut microbiota, and metabolic profiling. J. Hazard. Mater. 2022, 431, 128653. [Google Scholar] [CrossRef]
- De Silva, N.M.G.; Borges, M.C.; Hingorani, A.D.; Engmann, J.; Shah, T.; Zhang, X.S.; Luan, J.A.; Langenberg, C.; Wong, A.; Kuh, D.; et al. Liver Function and Risk of Type 2 Diabetes: Bidirectional Mendelian Randomization Study. Diabetes 2019, 68, 1681–1691. [Google Scholar] [CrossRef]
- Kavishankar, G.B.; Moree, S.S.; Lakshmidevi, N. Hepatoprotective and antioxidant activity of N-Trisaccharide in different experimental rats. Phytomedicine 2014, 21, 1026–1031. [Google Scholar] [CrossRef]
- Xu, X.; Dong, Y.; Ma, N.; Kong, W.; Yu, C.; Gong, L.; Chen, J.; Ren, J. MiR-337-3p lowers serum LDL-C level through targeting PCSK9 in hyperlipidemic mice. Metabolism 2021, 119, 154768. [Google Scholar] [CrossRef]
- Surakka, I.; Hornsby, W.E.; Farhat, L.; Rubenfire, M.; Fritsche, L.G.; Hveem, K.; Chen, Y.E.; Brook, R.D.; Willer, C.J.; Weinberg, R.L. A Novel Variant in APOB Gene Causes Extremely Low LDL-C Without Known Adverse Effects. JACC. Case Rep. 2020, 2, 775–779. [Google Scholar] [CrossRef]
- Wang, D.F.; Tan, K.S.; Zeng, W.P.; Li, S.X.; Wang, Y.Q.; Xu, F.P.; Tan, W. Hepatocellular BChE as a therapeutic target to ameliorate hypercholesterolemia through PRMT5 selective degradation to restore LDL receptor transcription. Life Sci. 2022, 293, 120336. [Google Scholar] [CrossRef]
- Bond, J.A.; Medinsky, M.A.; Dutcher, J.S. Metabolism of 1- 14C nitropyrene in isolated perfused rat livers. Toxicol. Appl. Pharmacol. 1984, 75, 531–538. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Ma, W.; Sun, X.; Fan, R.; Jin, Y.; Chen, N.; Zhu, X.; Guo, H.; Zhao, K.; et al. Diesel exhaust particles exposure induces liver dysfunction: Exploring predictive potential of human circulating microRNAs signature relevant to liver injury risk. J. Hazard. Mater. 2023, 458, 132060. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Yanagiba, Y.; Ramdhan, D.H.; Hayashi, Y.; Li, Y.F.; Suzuki, A.K.; Kamijima, M.; Nakajima, T. Nanoparticle-Rich Diesel Exhaust-Induced Liver Damage via Inhibited Transactivation of Peroxisome Proliferator-Activated Receptor Alpha. Environ. Toxicol. 2016, 31, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Hamade, A.K.; Misra, V.; Rabold, R.; Tankersley, C.G. Age-related changes in cardiac and respiratory adaptation to acute ozone and carbon black exposures: Interstrain variation in mice. Inhal. Toxicol. 2010, 22, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Slezakova, K.; Delerue-Matos, C.; Pereira, M.C.; Morais, S. Children environmental exposure to particulate matter and polycyclic aromatic hydrocarbons and biomonitoring in school environments: A review on indoor and outdoor exposure levels, major sources and health impacts. Environ. Int. 2019, 124, 180–204. [Google Scholar] [CrossRef]
- Salvi, S. Health effects of ambient air pollution in children. Paediatr. Respir. Rev. 2007, 8, 275–280. [Google Scholar] [CrossRef]
- Simioni, P.U.; Costa, E.H.; Tamashiro, W. Aging reduces the primary humoral response and the in vitro cytokine production in mice. Braz. J. Med. Biol. Res. 2007, 40, 1111–1120. [Google Scholar] [CrossRef]
- Muthumani, M.; Prabu, S.M. Silibinin potentially protects arsenic-induced oxidative hepatic dysfunction in rats. Toxicol. Mech. Methods 2012, 22, 277–288. [Google Scholar] [CrossRef]
- Li, Q.L.; Wu, Y.H.; Niu, M.; Lu, X.J.; Huang, Y.H.; He, D.H. Protective effects of tacalcitol against oxidative damage in human epidermal melanocytes. Int. J. Dermatol. 2017, 56, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.H.; Wu, Y.; Li, L.; Cai, Y.F.; Liu, M.; Gao, X.H.; Chen, H.D. Meta-analysis of the association between vitiligo and the level of superoxide dismutase or malondialdehyde. Clin. Exp. Dermatol. 2017, 42, 21–29. [Google Scholar] [CrossRef]
- Karsli, N.; Akcali, C.; Ozgoztasi, O.; Kirtak, N.; Inaloz, S. Role of oxidative stress in the pathogenesis of vitiligo with special emphasis on the antioxidant action of narrowband ultraviolet B phototherapy. J. Int. Med. Res. 2014, 42, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H.; Shih, T.S.; Chen, I.J.; Chen, H.L. Lipid Peroxidation and Oxidative Status Compared in Workers at a Bottom Ash Recovery Plant and Fly Ash Treatment Plants. J. Occup. Health 2008, 50, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.M.; Wang, G.L.; Bin, P.; Meng, T.; Niu, Y.; Yang, M.; Zhang, L.P.; Duan, H.W.; Yu, T.; Dai, Y.F.; et al. Time-course effects of antioxidants and phase II enzymes on diesel exhaust particles-induced oxidative damage in the mouse lung. Toxicol. Appl. Pharmacol. 2019, 366, 25–34. [Google Scholar] [CrossRef]
- Xu, M.M.; Wang, X.H.; Xu, L.; Zhang, H.; Li, C.F.; Liu, Q.; Chen, Y.Q.; Chung, K.F.; Adcock, I.M.; Li, F. Chronic lung inflammation and pulmonary fibrosis after multiple intranasal instillation of PM2.5 in mice. Environ. Toxicol. 2021, 36, 1434–1446. [Google Scholar] [CrossRef]
- Yin, F.; Driscoll, W.; Sulaiman, D.; Vergnes, L.; Ricks, J.; Ramanathan, G.; Stewart, J.; Mehrabian, M.; Beaven, S.W.; Reue, K.; et al. Diesel Exhaust Induces Mitochondrial Dysfunction, Hyperlipidemia and Liver Steatosis. Circulation 2018, 138, A17401. [Google Scholar] [CrossRef]
- Krivoshto, I.N.; Richards, J.R.; Albertson, T.E.; Derlet, R.W. The toxicity of diesel exhaust: Implications for primary care. J. Am. Board Fam. Med. 2008, 21, 55–62. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, International Agency for Research on Cancer. Diesel and gasoline engine exhausts and some nitroarenes. iarc monographs on the evaluation of carcinogenic risks to humans. IARC Monogr. Eval. Carcinog. Risks Hum. 2014, 105, 9–699. [Google Scholar]
- Tseng, C.Y.; Wang, J.S.; Chao, M.W. Causation by Diesel Exhaust Particles of Endothelial Dysfunctions in Cytotoxicity, Pro-inflammation, Permeability, and Apoptosis Induced by ROS Generation. Cardiovasc. Toxicol. 2017, 17, 384–392. [Google Scholar] [CrossRef]
- Wang, J.S.; Tseng, C.Y.; Chao, M.W. Diesel Exhaust Particles Contribute to Endothelia Apoptosis via Autophagy Pathway. Toxicol. Sci. 2017, 156, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Aquino, G.V.; Dabi, A.; Odom, G.J.; Zhang, F.; Bruce, E.D. Evaluating the endothelial-microglial interaction and comprehensive inflammatory marker profiles under acute exposure to ultrafine diesel exhaust particles in vitro. Toxicology 2021, 454, 152748. [Google Scholar] [CrossRef] [PubMed]
- Li, R.S.; Ning, Z.; Ai, L.S.; Sioutas, C.; Hsiai, T. Atmospheric Nanoparticles Induce Vascular Oxidative Stress via JNK Activation. Circulation 2008, 118, S318. [Google Scholar]



| PM2.5 (μg/m3) | CO (μg/m3) | NO (μg/m3) | NO2 (μg/m3) | |
|---|---|---|---|---|
| GE group | 50 | 25 | 2 | - |
| DE group | 370 | 50 | 20 | 4 |
| Control group | 6 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Zhang, X.; Li, X.; Zhang, J.; Lv, Z.; Guo, D.; Mao, H.; Wang, T. Lipid Dysregulation Induced by Gasoline and Diesel Exhaust Exposure and the Interaction with Age. Toxics 2024, 12, 303. https://doi.org/10.3390/toxics12040303
Gao Y, Zhang X, Li X, Zhang J, Lv Z, Guo D, Mao H, Wang T. Lipid Dysregulation Induced by Gasoline and Diesel Exhaust Exposure and the Interaction with Age. Toxics. 2024; 12(4):303. https://doi.org/10.3390/toxics12040303
Chicago/Turabian StyleGao, Yutong, Xinzhuo Zhang, Xinting Li, Jinsheng Zhang, Zongyan Lv, Dongping Guo, Hongjun Mao, and Ting Wang. 2024. "Lipid Dysregulation Induced by Gasoline and Diesel Exhaust Exposure and the Interaction with Age" Toxics 12, no. 4: 303. https://doi.org/10.3390/toxics12040303
APA StyleGao, Y., Zhang, X., Li, X., Zhang, J., Lv, Z., Guo, D., Mao, H., & Wang, T. (2024). Lipid Dysregulation Induced by Gasoline and Diesel Exhaust Exposure and the Interaction with Age. Toxics, 12(4), 303. https://doi.org/10.3390/toxics12040303