Lactobacillus acidophilus Fermented Dandelion Improves Hyperuricemia and Regulates Gut Microbiota
Abstract
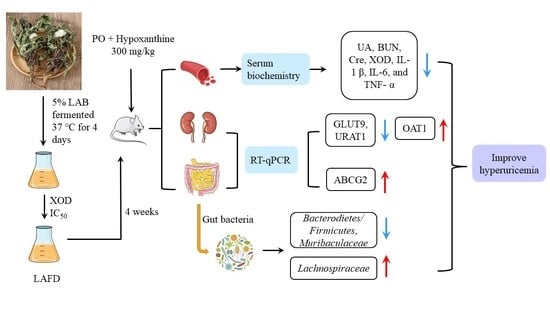
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fermented Dandelion
2.3. Determination of the Inhibitory Activity of XOD
2.4. Determination of Total Phenolic Content and Total Flavonoid Content
2.5. Antioxidant Activity Assay
2.5.1. Determination of DPPH Free Radical Scavenging Activity
2.5.2. Determination of ABTS Free Radical Scavenging Activity
2.5.3. Determination of Reducing Power
2.6. Animal Grouping, Modeling, and Administration
2.7. Serum Index Detection
2.8. Morphological Observation
2.9. qRT-PCR Analysis
2.10. Gut Microbiological Analysis
2.11. Statistical Analysis
3. Results
3.1. Determination of XOD Inhibitory Activity
3.2. Content and Antioxidant Activity Determination
3.3. Effects on Serum UA and XOD of Mice with HUA
3.4. Effect on Liver and Kidney Function of Mice with HUA
3.5. Effects on the Levels of Inflammatory Factors IL-1 β, IL-6, and TNF- α in Mice with HUA
3.6. Effects on Renal Pathological Changes in Mice with HUA
3.7. Effect on Pathological Changes of Ileum in Mice with HUA
3.8. Effects on the Levels of GLUT9, URAT1, OAT1 mRNA in Kidney and ABCG2 mRNA in Small Intestine in Mice with HUA
3.9. Effect on the Intestinal Microflora of Mice with HUA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9221. [Google Scholar] [CrossRef] [PubMed]
- Agnoletti, D.; Cicero, A.F.; Borghi, C. The Impact of Uric Acid and Hyperuricemia on Cardiovascular and Renal Systems. Cardiol. Clin. 2021, 39, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Stamp, L.K.; Chapman, P.T. Allopurinol hypersensitivity: Pathogenesis and prevention. Best Pr. Res. Clin. Rheumatol. 2020, 34, 101501. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Nasuhara, Y.; Momo, K.; Oki, H.; Kashiwagi, H.; Sato, Y.; Miyai, T.; Sugawara, M.; Takekuma, Y. Implementation Status of Liver Function Tests for Monitoring Benzbromarone-Induced Hepatotoxicity: An Epidemiological Survey Using the Japanese Claims Database. Biol. Pharm. Bull. 2021, 44, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhao, H.; Gao, Y.; Meng, Y.; Zhao, X.X.; Pan, S.N. Effects of Dandelion Extract on the Proliferation of Rat Skeletal Muscle Cells and the inhibition of a Lipopolysaccharide-lnduced Inflammatory Reaction. Chin. Med. J. 2018, 131, 1724–1731. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Y.; Wang, Z.; Chen, J.; Yang, Y.; Dong, G. Dandelion Extract Alleviated Lipopolysaccharide-Induced Oxidative Stress through the Nrf2 Pathway in Bovine Mammary Epithelial Cells. Toxins 2020, 12, 496. [Google Scholar] [CrossRef]
- Kang, L.; Miao, M.S.; Song, Y.G.; Fang, X.Y.; Zhang, J.; Zhang, Y.N.; Miao, J.X. Total flavonoids of Taraxacum mongolicum inhibit non-small cell lung cancer by regulating immune function. J. Ethnopharmacol. 2021, 281, 114514. [Google Scholar] [CrossRef]
- Wirngo, F.E.; Lambert, M.N.; Jeppesen, P.B. The Physiological Effects of Dandelion (Taraxacum officinale) in Type 2 Diabetes. Rev. Diabet. Stud. RDS 2016, 13, 113–131. [Google Scholar] [CrossRef] [Green Version]
- Aabideen, Z.U.; Mumtaz, M.W.; Akhtar, M.T.; Mukhtar, H.; Raza, S.A.; Touqeer, T.; Saari, N. Anti-Obesity Attributes; UHPLC-QTOF-MS/MS-Based Metabolite Profiling and Molecular Docking Insights of Taraxacum officinale. Molecules 2020, 25, 4935. [Google Scholar] [CrossRef]
- Hosseini Shekarabi, S.P.; Mostafavi, Z.S.; Mehrgan, M.S.; Islami, H.R. Dietary supplementation with dandelion (Taraxacum officinale) flower extract provides immunostimulation and resistance against Streptococcus iniae infection in rainbow trout (Oncorhynchusmykiss). Fish Shellfish Immunol. 2021, 118, 180–187. [Google Scholar] [CrossRef]
- Zou, C.Z.; Wang, H.J.; Li, J.; Liu, Z.; Li, Y.T.; Ma, H.X. Effect of Pugongying(Taraxacum mongolicum Hand.-Mazz.) on Acute Hyperuricemia Rats(in Chinese). Chin. Arch. Tradit. Chin. Med. 2020, 38, 170–172. [Google Scholar] [CrossRef]
- Zou, C.Z.; Wang, H.J.; Li, J.; Liu, Z.; Li, Y.T.; Ma, H.X. The Protection of T. Hand.-Mazz. on Renal Injury Rats with Persistent Hyperuricemia(in Chinese). Chin. J. Ethnomedicine Ethnopharmacy 2020, 29, 10–12. [Google Scholar]
- Li, L.; Wang, L.; Fan, W.; Jiang, Y.; Zhang, C.; Li, J.; Peng, W.; Wu, C. The Application of Fermentation Technology in Traditional Chinese Medicine: A Review. Am. J. Chin. Med. 2020, 48, 899–921. [Google Scholar] [CrossRef] [PubMed]
- Gadhoumi, H.; Hayouni, E.L.A.; Martinez-Rojas, E.; Yeddes, W.; Tounsi, M.S. Biochemical composition, antimicrobial and antifungal activities assessment of the fermented medicinal plants extract using lactic acid bacteria. Arch. Microbiol. 2022, 204, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Eweys, A.S.; Zhao, Y.-S.; Darwesh, O.M. Improving the antioxidant and anticancer potential of Cinnamomum cassia via fermentation with Lactobacillus plantarum. Biotechnol. Rep. (Amst. Neth.) 2022, 36, e00768. [Google Scholar] [CrossRef]
- Oh, N.S.; Lee, J.Y.; Lee, J.M.; Lee, K.W.; Kim, Y. Mulberry leaf extract fermented with Lactobacillus acidophilus A4 ameliorates 5-fluorouracil-induced intestinal mucositis in rats. Lett. Appl. Microbiol. 2017, 64, 459–468. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, D.; Shen, Y.; Chen, Y.; Qian, J.; Fu, G. Effect of Lactobacillus acidophilus fermentation on the composition of chlorogenic acids and anti-hyperuricemia activity of Artemisia selengensis Turcz. Food Funct. 2022, 13, 11780–11793. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Wu, J.; Ou, K.; Huang, Q.; Cao, J.; Duan, T.; Zhou, L.; Pan, Y. Chemical profile and antioxidant activity of bidirectional metabolites from Tremella fuciformis and Acanthopanax trifoliatus as assessed using response surface methodology. Front. Nutr. 2022, 9, 1035788. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, Q.; Hu, L.; Liu, T.; Zheng, B.; Lu, D.; Guo, C.; Zhou, L. Enhanced exopolysaccharide yield and antioxidant activities of Schizophyllum commune fermented products by the addition of Radix Puerariae. RSC Adv. 2021, 11, 38219–38234. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, H.; Huang, Q.; Tu, L.; Hu, L.; Zheng, B.; Sun, H.; Lu, D.; Guo, C.; Zhou, L. Mechanism of Longevity Extension of Caenorhabditis elegans Induced by Schizophyllum commune Fermented Supernatant With Added Radix Puerariae. Front. Nutr. 2022, 9, 847064. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, G.; Liao, Y.; Pan, J. Inhibition of chrysin on xanthine oxidase activity and its inhibition mechanism. Int. J. Biol. Macromol. 2015, 81, 274–282. [Google Scholar] [CrossRef]
- Liu, Y.; Han, C.; Lu, T.; Liu, Y.; Chen, H.; Yang, C.; Tu, Y.; Li, Y. Investigation of the interaction between Chrysoeriol and xanthine oxidase using computational and in vitro approaches. Int. J. Biol. Macromol. 2021, 190, 463–473. [Google Scholar] [CrossRef]
- Wang, Z.N.; Feng, Y.Z.; Yang, N.N.; Jiang, T.; Xu, H.D.; Lei, H.J. Fermentation of kiwifruit juice from two cultivars by probiotic bacteria: Bioactive phenolics, antioxidant activities and flavor volatiles. Food Chem. 2022, 373, 131455. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Wang, Y.; Ren, X.R.; Zhao, Y.; Liu, N.; An, X.P.; Qi, J.W. Isolation and Characterization of Flavonoids from Fermented Dandelion (Taraxacum mongolicum Hand.-Mazz.), and Assessment of Its Antioxidant Actions In Vitro and In Vivo. Fermentation 2022, 8, 306. [Google Scholar] [CrossRef]
- Xie, J.H.; Zhang, F.; Wang, Z.J.; Shen, M.Y.; Nie, S.P.; Xie, M.Y. Preparation, characterization and antioxidant activities of acetylated polysaccharides from Cyclocarya paliurus leaves. Carbohydr. Polym. 2015, 133, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.T.; Yusof, Y.A.; Mokhtar, M.N.; Ya’acob, M.E.; Mohd Ghazali, H.; Chang, L.S.; Manaf, Y.N. Coconut (Cocos nucifera L.) sap as a potential source of sugar: Antioxidant and nutritional properties. Food Sci. Nutr. 2020, 8, 1777–1787. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Huang, H.; Huang, G. Extraction, derivatization and antioxidant activity of cucumber polysaccharide. Int. J. Biol. Macromol. 2019, 140, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jin, X.; Zhang, X.; Xie, X.; Tu, Z.; He, X. From Function to Metabolome: Metabolomic Analysis Reveals the Effect of Probiotic Fermentation on the Chemical Compositions and Biological Activities of Perilla frutescens Leaves. Front. Nutr. 2022, 9, 933193. [Google Scholar] [CrossRef] [PubMed]
- BM, A.L.; Elgebaly, H.A.; Germoush, M.O.; Qarmush, M.M.; Azab, M.S.; Alruhaimi, R.S.; Ahmeda, A.F.; Abukhalil, M.H.; Kamel, E.M.; Arab, H.H.; et al. A flavonoid-rich fraction of Monolluma quadrangula inhibits xanthine oxidase and ameliorates potassium oxonate-induced hyperuricemia in rats. Env. Sci. Pollut. Res. Int. 2022, 29, 63520–63532. [Google Scholar] [CrossRef]
- Huang, L.; He, X.; Peng, W.; He, X.; Xu, B.; Xu, H.; Wang, Y.; Xu, W.; Chen, W.; Wang, S.; et al. Hyperuricemia induces liver injury by upregulating HIF-1α and inhibiting arginine biosynthesis pathway in mouse liver and human L02 hepatocytes. Biochem. Biophys. Res. Commun. 2022, 617, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Kim, D.S. Synergistic Impacts of Alpinia oxyphylla Seed Extract and Allopurinol against Experimental Hyperuricemia. Biomed Res. Int. 2022, 2022, 2824535. [Google Scholar] [CrossRef]
- Wang, M.; Lin, X.; Yang, X.; Yang, Y. Research progress on related mechanisms of uric acid activating NLRP3 inflammasome in chronic kidney disease. Ren. Fail. 2022, 44, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Ohno, I. Relationship between hyperuricemia and chronic kidney disease. Nucl. Nucl. Nucleic Acids 2011, 30, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Liu, Q.; Hao, H.; Bu, Y.; Tian, X.; Wang, T.; Yi, H. Lactobacillus paracasei X11 Ameliorates Hyperuricemia and Modulates Gut Microbiota in Mice. Front. Immunol. 2022, 13, 940228. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Sun, S.; Huang, Y.; Gao, Q.; Xie, X.; Wang, P.; Li, J.; Liang, L.; He, X.; Jiang, Y.; et al. Metagenomic analysis revealed the potential role of gut microbiome in gout. NPJ Biofilms Microbiomes 2021, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Werlinger, P.; Suh, J.W.; Cheng, J. Potential Probiotic Lacticaseibacillus paracasei MJM60396 Prevents Hyperuricemia in a Multiple Way by Absorbing Purine, Suppressing Xanthine Oxidase and Regulating Urate Excretion in Mice. Microorganisms 2022, 10, 851. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Lu, Z.X.; Lu, Y.J. The potential of probiotics in the amelioration of hyperuricemia. Food Funct. 2022, 13, 2394–2414. [Google Scholar] [CrossRef]
- Xu, J.; Xu, J.; Shi, T.; Zhang, Y.; Chen, F.; Yang, C.; Guo, X.; Liu, G.; Shao, D.; Leong, K.W.; et al. Probiotic-inspired nanomedicine restores intestinal homeostasis in colitis by regulating redox balance, immune responses, and the gut microbiome. Adv. Mater. 2022, 35, e2207890. [Google Scholar] [CrossRef]
- Wu, Y.; Jha, R.; Li, A.; Liu, H.; Zhang, Z.; Zhang, C.; Zhai, Q.; Zhang, J. Probiotics (Lactobacillus plantarum HNU082) Supplementation Relieves Ulcerative Colitis by Affecting Intestinal Barrier Functions, Immunity-Related Gene Expression, Gut Microbiota, and Metabolic Pathways in Mice. Microbiol. Spectr. 2022, 10, e0165122. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Xu, L.; Fang, X.; Wan, Y.; Yu, D.; Guo, Y. A tetrapeptide from maize combined with probiotics exerted strong anti-inflammatory effects and modulated gut microbiota in DSS-induced colitis mice. Food Funct. 2022, 13, 12602–12618. [Google Scholar] [CrossRef]
- James, A.; Ke, H.M.; Yao, T.; Wang, Y.S. The Role of Probiotics in Purine Metabolism, Hyperuricemia and Gout: Mechanisms and Interventions. Food Rev. Int. 2023, 39, 261–277. [Google Scholar] [CrossRef]
- Hsieh, M.W.; Chen, H.Y.; Tsai, C.C. Screening and Evaluation of Purine-Nucleoside-Degrading Lactic Acid Bacteria Isolated from Winemaking Byproducts In Vitro and Their Uric Acid-Lowering Effects In Vivo. Fermentation 2021, 7, 74. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, C.; Zeng, X.; Yuan, Z. Microecological treatment of hyperuricemia using Lactobacillus from pickles. BMC Microbiol. 2020, 20, 195. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Cheng, F.; Li, W.; Yu, Q.; Ma, C.; Zou, Y.; Xu, T.; Liu, S.; Zhang, S.; Wang, Q. Enhancement of anti-inflammatory effect of cattle bile by fermentation and its inhibition of neuroinflammation on microglia by inhibiting NLRP3 inflammasome. J. Biosci. Bioeng. 2022, 133, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Su, Y.; An, Z.; Zhang, P.; Yue, Q.; Zhao, C.; Sun, X.; Zhang, S.; Liu, X.; Li, K.; et al. Fermentation products of Danshen relieved dextran sulfate sodium-induced experimental ulcerative colitis in mice. Sci. Rep. 2021, 11, 16210. [Google Scholar] [CrossRef]
- González-Castejón, M.; Visioli, F.; Rodriguez-Casado, A. Diverse biological activities of dandelion. Nutr. Rev. 2012, 70, 534–547. [Google Scholar] [CrossRef]
- Jhang, J.J.; Ong, J.W.; Lu, C.C.; Hsu, C.L.; Lin, J.H.; Liao, J.W.; Yen, G.C. Hypouricemic effects of Mesona procumbens Hemsl. through modulating xanthine oxidase activity in vitro and in vivo. Food Funct. 2016, 7, 4239–4246. [Google Scholar] [CrossRef]
- Gao, H.; Li, X.; Chen, X.; Hai, D.; Wei, C.; Zhang, L.; Li, P. The Functional Roles of Lactobacillus acidophilus in Different Physiological and Pathological Processes. J. Microbiol. Biotechnol. 2022, 32, 1226–1233. [Google Scholar] [CrossRef]
- Kim, H.; Nam, B.Y.; Park, J.; Song, S.; Kim, W.K.; Lee, K.; Nam, T.W.; Park, J.T.; Yoo, T.H.; Kang, S.W.; et al. Lactobacillus acidophilus KBL409 Reduces Kidney Fibrosis via Immune Modulatory Effects in Mice with Chronic Kidney Disease. Mol. Nutr. Food Res. 2022, 66, 2101105. [Google Scholar] [CrossRef]
- Luis-Rodriguez, D.; Donate-Correa, J.; Martin-Nunez, E.; Ferri, C.; Tagua, V.G.; Castro, A.P.; Mora-Fernandez, C.; Navarro-Gonzalez, J.F. Serum urate is related to subclinical inflammation in asymptomatic hyperuricaemia. Rheumatology 2021, 60, 371–379. [Google Scholar] [CrossRef]
- Kluck, V.; Liu, R.Q.; Joosten, L.A.B. The role of interleukin-1 family members in hyperuricemia and gout. Jt. Bone Spine 2021, 88, 105092. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Nie, Y.; Chang, Y.; Zeng, S.; Liang, C.; Zheng, X.; Xiao, D.; Zhan, S.; Zheng, Q. Protective effects of Rhizoma smilacis glabrae extracts on potassium oxonate- and monosodium urate-induced hyperuricemia and gout in mice. Phytomedicine 2019, 59, 152772. [Google Scholar] [CrossRef]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular identification of a renal urate-anion exchanger that regulates blood urate levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Kim, G.-H. Urate Transporters in the Kidney: What Clinicians Need to Know. Electrolyte Blood Press. 2021, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.X.; Li, C.H.; Zhou, P.; Jiang, T.L. Uric acid transporters hiding in the intestine. Pharm. Biol. 2016, 54, 3151–3155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchiando, A.M.; Shen, L.; Graham, W.V.; Edelblum, K.L.; Duckworth, C.A.; Guan, Y.F.; Montrose, M.H.; Turner, J.R.; Watson, A.J.M. The Epithelial Barrier Is Maintained by In Vivo Tight Junction Expansion During Pathologic Intestinal Epithelial Shedding. Gastroenterology 2011, 140, 1208–1218. [Google Scholar] [CrossRef] [Green Version]
- Eckenstaler, R.; Benndorf, R.A. The Role of ABCG2 in the Pathogenesis of Primary Hyperuricemia and Gout-An Update. Int. J. Mol. Sci. 2021, 22, 6678. [Google Scholar] [CrossRef]
- Liu, T.; Gao, H.; Zhang, Y.; Wang, S.; Lu, M.; Dai, X.; Liu, Y.; Shi, H.; Xu, T.; Yin, J.; et al. Apigenin Ameliorates Hyperuricemia and Renal Injury through Regulation of Uric Acid Metabolism and JAK2/STAT3 Signaling Pathway. Pharmaceuticals 2022, 15, 1442. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Z.; Zhang, B.; Nie, A.; Bian, M. Cichorium intybus L. promotes intestinal uric acid excretion by modulating ABCG2 in experimental hyperuricemia. Nutr. Metab. 2017, 14, 38. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Sun, J.; Zhang, Y.; Shao, T.J.; Li, H.C.; Wang, M.J.; Zhang, L.; Bian, H.; Wen, C.P.; Xie, Z.J.; et al. Effect of a Traditional Chinese Medicine Formula (CoTOL) on Serum Uric Acid and Intestinal Flora in Obese Hyperuricemic Mice Inoculated with Intestinal Bacteria. Evid.-Based Complement. Altern. Med. 2020, 2020, 8831937. [Google Scholar] [CrossRef]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 2017, 15, 630–638. [Google Scholar] [CrossRef]
- Wang, Z.L.; Li, Y.C.; Liao, W.H.; Huang, J.; Liu, Y.P.; Li, Z.Y.; Tang, J.Y. Gut microbiota remodeling: A promising therapeutic strategy to confront hyperuricemia and gout. Front. Cell. Infect. Microbiol. 2022, 12, 935723. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.; Wang, Z.; Ang, K.Y.; Huang, S.; Hou, Q.; Su, X.; Qiao, J.; Zheng, Y.; Wang, L.; et al. Intestinal Microbiota Distinguish Gout Patients from Healthy Humans. Sci. Rep. 2016, 6, 20602. [Google Scholar] [CrossRef] [Green Version]
- Haller, D.; Hoermannsperger, G. Interaction between humans and intestinal bacteria as a determinant for intestinal health. Intestinal microbiome and inflammatory bowel diseases. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz 2015, 58, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Martinez, I.; Walter, J.; Keshavarzian, A.; Rose, D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 2013, 23, 74–81. [Google Scholar] [CrossRef]
- Marzocco, S.; Fazeli, G.; Di Micco, L.; Autore, G.; Adesso, S.; Dal Piaz, F.; Heidland, A.; Di Iorio, B. Supplementation of Short-Chain Fatty Acid, Sodium Propionate, in Patients on Maintenance Hemodialysis: Beneficial Effects on Inflammatory Parameters and Gut-Derived Uremic Toxins, A Pilot Study (PLAN Study). J. Clin. Med. 2018, 7, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fei, N.; Zhao, L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013, 7, 880–884. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Liu, C.; Jiang, Q.; Yin, Y. Butyrate in Energy Metabolism: There Is Still More to Learn. Trends Endocrinol. Metab. TEM 2021, 32, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Han, P.; Ma, S.; Peng, R.; Wang, C.; Kong, W.; Cong, L.; Fu, J.; Zhang, Z.; Yu, H.; et al. Abnormal metabolism of gut microbiota reveals the possible molecular mechanism of nephropathy induced by hyperuricemia. Acta Pharm. Sinica B 2020, 10, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| GAPDH | CCTCGTCCCGTAGACAAAATG | TGAGGTCAATGAAGGGGTCGT |
| GLUT9 | ATGGTCCTTCTCGCTCGTCG | TATCCGGGTCAATGGGCTGT |
| URAT1 | CGCTTCCGACAACCTCAAT | GAGTTACATACCAGGTCCCACG |
| OAT1 | TGTGCTTCCTAGTCATCAATTCCA | CAGGGATGTGCGAATGATTGTA |
| ABCG2 | TTGTCCAGGATTCAATGTAACGG | TGACAGTTCGATGCCCTGATTT |
| Drug | IC50 (mg/mL) |
|---|---|
| Dandelion | 19.80 ± 1.23 a |
| Lactic acid bacteria 1.648 fermentation dandelion | 20.33 ± 1.79 a |
| Lactic acid bacteria 1.191 fermentation dandelion | 18.45 ± 1.19 ab |
| Lactic acid bacteria 1.140 fermentation dandelion | 19.13 ± 0.98 a |
| Lactobacillus casei fermentation dandelion | 16.10 ± 2.30 b |
| Lactobacillus rhamnosus fermentation dandelion | 19.47 ± 0.78 a |
| Lactobacillus acidophilus fermentation dandelion | 15.55 ± 2.31 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Chen, M.; Liu, Y.; Tong, Y.; Liu, T.; Wu, L.; Wang, J.; Han, B.; Zhou, L.; Hu, X. Lactobacillus acidophilus Fermented Dandelion Improves Hyperuricemia and Regulates Gut Microbiota. Fermentation 2023, 9, 352. https://doi.org/10.3390/fermentation9040352
Ma Q, Chen M, Liu Y, Tong Y, Liu T, Wu L, Wang J, Han B, Zhou L, Hu X. Lactobacillus acidophilus Fermented Dandelion Improves Hyperuricemia and Regulates Gut Microbiota. Fermentation. 2023; 9(4):352. https://doi.org/10.3390/fermentation9040352
Chicago/Turabian StyleMa, Qianwen, Mingju Chen, Yu Liu, Ying Tong, Tianfeng Liu, Lele Wu, Jiliang Wang, Bin Han, Lin Zhou, and Xuguang Hu. 2023. "Lactobacillus acidophilus Fermented Dandelion Improves Hyperuricemia and Regulates Gut Microbiota" Fermentation 9, no. 4: 352. https://doi.org/10.3390/fermentation9040352
APA StyleMa, Q., Chen, M., Liu, Y., Tong, Y., Liu, T., Wu, L., Wang, J., Han, B., Zhou, L., & Hu, X. (2023). Lactobacillus acidophilus Fermented Dandelion Improves Hyperuricemia and Regulates Gut Microbiota. Fermentation, 9(4), 352. https://doi.org/10.3390/fermentation9040352