Current Trends in Xenobiotic Sensing Nuclear Receptor Research—The Many Facets of PXR and CAR
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cell Signaling".
Viewed by 55852
Share This Topical Collection
Editors
 Dr. Oliver Burk
Dr. Oliver Burk
 Dr. Oliver Burk
Dr. Oliver Burk
E-Mail
Website
Collection Editor
Nuclear receptors and pharmacogenomics, Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Auerbachstrasse 112, 70376 Stuttgart, Germany
Interests: nuclear receptors; molecular mechanisms of gene regulation; drug metabolism and transport; drug-induced resistance of cancer cells; non-alcoholic fatty liver disease; pharmacogenomics
 Prof. Dr. Björn Windshügel
Prof. Dr. Björn Windshügel
 Prof. Dr. Björn Windshügel
Prof. Dr. Björn Windshügel
E-Mail
Website
Collection Editor
Fraunhofer Institute for Translational Medicine and Pharmacology ITMP, ScreeningPort, Schnackenburgallee 114, 22525 Hamburg, Germany
Interests: nuclear receptors; drug discovery; nuclear receptor modulators; development of cancer therapeutics
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
The two closely related xenosensing nuclear receptors PXR (pregnane X receptor, NR1I2) and CAR (constitutive androstane receptor, NR1I3) are ligand-modulated master regulators of drug metabolism and transport. By enhancing the capacity of the body to detoxify and eliminate small-molecule drugs, their activation plays a central role in drug–drug interactions leading to adverse reactions or loss of therapeutic efficacy. In addition, PXR and CAR play distinct roles in the regulation of basic cell metabolic pathways, such as glucose and lipid metabolism, as well as in cancer cell proliferation and tumorigenesis. Due to their manifold roles, the development of both agonists and antagonists as potential therapeutics against metabolic diseases and cancer is of great interest.
In order to highlight recent findings in PXR and CAR research, this Topical Collection seeks manuscript submissions for original and review articles from all research disciplines. Topics may cover but are not limited to the discovery and characterization of novel ligands, structural elucidations, investigations on the role of PXR and CAR in cancer development and therapy, assessment of PXR- and/or CAR-modulated metabolic and signaling pathways, or the development of novel techniques and assays for studying receptor function.
We look forward to your contribution.
Dr. Oliver Burk
Prof. Dr. Björn Windshügel
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- ligands
- screening
- molecular modelling
- cheminformatics
- ADME
- tumorigenesis
- chemoresistance
- signaling
- metabolism
- mechanism of gene regulation
Published Papers (14 papers)
Open AccessReview
Pregnane X Receptor Signaling Pathway and Vitamin K: Molecular Mechanisms and Clinical Relevance in Human Health
by
Jeff L. Staudinger, Avina Mahroke, Gauri Patel, Cole Dattel and Sahana Reddy
Cited by 3 | Viewed by 2679
Abstract
This review explores the likely clinical impact of Pregnane X Receptor (PXR) activation by vitamin K on human health. PXR, initially recognized as a master regulator of xenobiotic metabolism in liver, emerges as a key regulator influencing intestinal homeostasis, inflammation, oxidative stress, and
[...] Read more.
This review explores the likely clinical impact of Pregnane X Receptor (PXR) activation by vitamin K on human health. PXR, initially recognized as a master regulator of xenobiotic metabolism in liver, emerges as a key regulator influencing intestinal homeostasis, inflammation, oxidative stress, and autophagy. The activation of PXR by vitamin K highlights its role as a potent endogenous and local agonist with diverse clinical implications. Recent research suggests that the vitamin K-mediated activation of PXR highlights this vitamin’s potential in addressing pathophysiological conditions by promoting hepatic detoxification, fortifying gut barrier integrity, and controlling pro-inflammatory and apoptotic pathways. PXR activation by vitamin K provides an intricate association with cancer cell survival, particularly in colorectal and liver cancers, to provide new insights into potential novel therapeutic strategies. Understanding the clinical implications of PXR activation by vitamin K bridges molecular mechanisms with health outcomes, further offering personalized therapeutic approaches for complex diseases.
Full article
►▼
Show Figures
Open AccessArticle
New Approach Methods for Hazard Identification: A Case Study with Azole Fungicides Affecting Molecular Targets Associated with the Adverse Outcome Pathway for Cholestasis
by
Constanze Knebel, Roderich D. Süssmuth, Helen S. Hammer, Albert Braeuning and Philip Marx-Stoelting
Cited by 3 | Viewed by 1967
Abstract
Triazole fungicides such as propiconazole (Pi) or tebuconazole (Te) show hepatotoxicity in vivo, e.g., hypertrophy and vacuolization of liver cells following interaction with nuclear receptors such as PXR (pregnane-X-receptor) and CAR (constitutive androstane receptor). Accordingly, azoles affect gene expression associated with these adverse
[...] Read more.
Triazole fungicides such as propiconazole (Pi) or tebuconazole (Te) show hepatotoxicity in vivo, e.g., hypertrophy and vacuolization of liver cells following interaction with nuclear receptors such as PXR (pregnane-X-receptor) and CAR (constitutive androstane receptor). Accordingly, azoles affect gene expression associated with these adverse outcomes in vivo but also in human liver cells in vitro. Additionally, genes indicative of liver cholestasis are affected in vivo and in vitro. We therefore analyzed the capability of Pi and Te to cause cholestasis in an adverse outcome pathway (AOP)-driven approach in hepatic cells of human origin in vitro, considering also previous in vivo studies. Bile salt export pump (BSEP) activity assays confirmed that both azoles are weak inhibitors of BSEP. They alternate the expression of various cholestasis-associated target genes and proteins as well as the mitochondrial membrane function. Published in vivo data, however, demonstrate that neither Pi nor Te cause cholestasis in rodent bioassays. This discrepancy can be explained by the in vivo concentrations of both azoles being well below their EC50 for BSEP inhibition. From a regulatory perspective, this illustrates that toxicogenomics and human in vitro models are valuable tools to detect the potential of a substance to cause a specific type of toxicity. To come to a sound regulatory conclusion on the in vivo relevance of such a finding, results will have to be considered in a broader context also including toxicokinetics in a weight-of-evidence approach.
Full article
►▼
Show Figures
Open AccessArticle
Combination of Paclitaxel and PXR Antagonist SPA70 Reverses Paclitaxel-Resistant Non-Small Cell Lung Cancer
by
Xiaxia Niu, Ting Wu, Qishuang Yin, Xinsheng Gu, Gege Li, Changlong Zhou, Mei Ma, Li Su, Shu Tang, Yanan Tian, Ming Yang and Hongmei Cui
Cited by 10 | Viewed by 2719
Abstract
Paclitaxel (PTX) is one of the most efficient drugs for late-stage non-small cell lung cancer (NSCLC) patients. However, most patients gradually develop resistance to PTX with long-term treatments. The identification of new strategies to reverse PTX resistance in NSCLC is crucially important for
[...] Read more.
Paclitaxel (PTX) is one of the most efficient drugs for late-stage non-small cell lung cancer (NSCLC) patients. However, most patients gradually develop resistance to PTX with long-term treatments. The identification of new strategies to reverse PTX resistance in NSCLC is crucially important for the treatment. PTX is an agonist for the pregnane X receptor (PXR) which regulates PTX metabolism. Antagonizing PXR, therefore, may render the NSCLC more sensitive to the PTX treatment. In this study, we investigated the PXR antagonist SPA70 and its role in PTX treatment of NSCLC. In vitro, SPA70 and PTX synergistically inhibited cell growth, migration and invasion in both paclitaxel-sensitive and paclitaxel-resistant A549 and H460 lung cancer cells. Mechanistically, we found PTX and SPA70 cotreatment disassociated PXR from ABCB1 (MDR1, P-gp) promoter, thus inhibiting P-gp expression. Furthermore, the combination regimen synergistically enhanced the interaction between PXR and Tip60, which abrogated Tip60-mediated α-tubulin acetylation, leading to mitosis defect, S-phase arrest and necroptosis/apoptosis. Combination of PXT and SPA70 dramatically inhibited tumor growth in a paclitaxel-resistant A549/TR xenograft tumor model. Taken together, we showed that SPA70 reduced the paclitaxel resistance of NSCLC. The combination regimen of PTX and SPA70 could be potential novel candidates for the treatment of taxane-resistant lung cancer.
Full article
►▼
Show Figures
Open AccessReview
Allosteric Antagonism of the Pregnane X Receptor (PXR): Current-State-of-the-Art and Prediction of Novel Allosteric Sites
by
Rajamanikkam Kamaraj, Martin Drastik, Jana Maixnerova and Petr Pavek
Cited by 6 | Viewed by 4311
Abstract
The pregnane X receptor (PXR,
NR1I2) is a xenobiotic-activated transcription factor with high levels of expression in the liver. It not only plays a key role in drug metabolism and elimination, but also promotes tumor growth, drug resistance, and metabolic diseases. It
[...] Read more.
The pregnane X receptor (PXR,
NR1I2) is a xenobiotic-activated transcription factor with high levels of expression in the liver. It not only plays a key role in drug metabolism and elimination, but also promotes tumor growth, drug resistance, and metabolic diseases. It has been proposed as a therapeutic target for type II diabetes, metabolic syndrome, and inflammatory bowel disease, and PXR antagonists have recently been considered as a therapy for colon cancer. There are currently no PXR antagonists that can be used in a clinical setting. Nevertheless, due to the large and complex ligand-binding pocket (LBP) of the PXR, it is challenging to discover PXR antagonists at the orthosteric site. Alternative ligand binding sites of the PXR have also been proposed and are currently being studied. Recently, the AF-2 allosteric binding site of the PXR has been identified, with several compounds modulating the site discovered. Herein, we aimed to summarize our current knowledge of allosteric modulation of the PXR as well as our attempt to unlock novel allosteric sites. We describe the novel binding function 3 (BF-3) site of PXR, which is also common for other nuclear receptors. In addition, we also mention a novel allosteric site III based on in silico prediction. The identified allosteric sites of the PXR provide new insights into the development of safe and efficient allosteric modulators of the PXR receptor. We therefore propose that novel PXR allosteric sites might be promising targets for treating chronic metabolic diseases and some cancers.
Full article
►▼
Show Figures
Open AccessArticle
Target Hopping from Protein Kinases to PXR: Identification of Small-Molecule Protein Kinase Inhibitors as Selective Modulators of Pregnane X Receptor from TüKIC Library
by
Enni-Kaisa Mustonen, Tatu Pantsar, Azam Rashidian, Juliander Reiner, Matthias Schwab, Stefan Laufer and Oliver Burk
Cited by 3 | Viewed by 4135
Abstract
Small-molecule protein kinase inhibitors are used for the treatment of cancer, but off-target effects hinder their clinical use. Especially off-target activation of the pregnane X receptor (PXR) has to be considered, as it not only governs drug metabolism and elimination, but also can
[...] Read more.
Small-molecule protein kinase inhibitors are used for the treatment of cancer, but off-target effects hinder their clinical use. Especially off-target activation of the pregnane X receptor (PXR) has to be considered, as it not only governs drug metabolism and elimination, but also can promote tumor growth and cancer drug resistance. Consequently, PXR antagonism has been proposed for improving cancer drug therapy. Here we aimed to identify small-molecule kinase inhibitors of the Tübingen Kinase Inhibitor Collection (TüKIC) compound library that would act also as PXR antagonists. By a combination of in silico screen and confirmatory cellular reporter gene assays, we identified four novel PXR antagonists and a structurally related agonist with a common phenylaminobenzosuberone scaffold. Further characterization using biochemical ligand binding and cellular protein interaction assays classified the novel compounds as mixed competitive/noncompetitive, passive antagonists, which bind PXR directly and disrupt its interaction with coregulatory proteins. Expression analysis of prototypical PXR target genes ABCB1 and CYP3A4 in LS174T colorectal cancer cells and HepaRG hepatocytes revealed novel antagonists as selective receptor modulators, which showed gene- and tissue-specific effects. These results demonstrate the possibility of dual PXR and protein kinase inhibitors, which might represent added value in cancer therapy.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
Development and Experimental Validation of Regularized Machine Learning Models Detecting New, Structurally Distinct Activators of PXR
by
Steffen Hirte, Oliver Burk, Ammar Tahir, Matthias Schwab, Björn Windshügel and Johannes Kirchmair
Cited by 3 | Viewed by 2981
Abstract
The pregnane X receptor (PXR) regulates the metabolism of many xenobiotic and endobiotic substances. In consequence, PXR decreases the efficacy of many small-molecule drugs and induces drug-drug interactions. The prediction of PXR activators with theoretical approaches such as machine learning (ML) proves challenging
[...] Read more.
The pregnane X receptor (PXR) regulates the metabolism of many xenobiotic and endobiotic substances. In consequence, PXR decreases the efficacy of many small-molecule drugs and induces drug-drug interactions. The prediction of PXR activators with theoretical approaches such as machine learning (ML) proves challenging due to the ligand promiscuity of PXR, which is related to its large and flexible binding pocket. In this work we demonstrate, by the example of random forest models and support vector machines, that classifiers generated following classical training procedures often fail to predict PXR activity for compounds that are dissimilar from those in the training set. We present a novel regularization technique that penalizes the gap between a model’s training and validation performance. On a challenging test set, this technique led to improvements in Matthew correlation coefficients (MCCs) by up to 0.21. Using these regularized ML models, we selected 31 compounds that are structurally distinct from known PXR ligands for experimental validation. Twelve of them were confirmed as active in the cellular PXR ligand-binding domain assembly assay and more hits were identified during follow-up studies. Comprehensive analysis of key features of PXR biology conducted for three representative hits confirmed their ability to activate the PXR.
Full article
►▼
Show Figures
Open AccessArticle
Pregnane X Receptor Mediates Atherosclerosis Induced by Dicyclohexyl Phthalate in LDL Receptor-Deficient Mice
by
Jingwei Liu, Rebecca Hernandez, Xiuchun Li, Zhaojie Meng, Hong Chen and Changcheng Zhou
Cited by 10 | Viewed by 3326
Abstract
Plastic-associated endocrine disrupting chemicals (EDCs) have been implicated in the etiology of cardiovascular disease (CVD) in humans, but the underlying mechanisms remain elusive. Dicyclohexyl phthalate (DCHP) is a widely used phthalate plasticizer; whether and how exposure to DCHP elicits adverse effects in vivo
[...] Read more.
Plastic-associated endocrine disrupting chemicals (EDCs) have been implicated in the etiology of cardiovascular disease (CVD) in humans, but the underlying mechanisms remain elusive. Dicyclohexyl phthalate (DCHP) is a widely used phthalate plasticizer; whether and how exposure to DCHP elicits adverse effects in vivo is mostly unknown. We previously reported that DCHP is a potent ligand of the pregnane X receptor (PXR) which acts as a xenobiotic sensor to regulate xenobiotic metabolism. PXR also functions in macrophages to regulate atherosclerosis development in animal models. In the current study, LDL receptor-deficient mice with myeloid-specific PXR deficiency (PXR
ΔMyeLDLR
−/−) and their control littermates (PXR
F/FLDLR
−/−) were used to determine the impact of DCHP exposure on macrophage function and atherosclerosis. Chronic exposure to DCHP significantly increased atherosclerotic lesion area in the aortic root and brachiocephalic artery of PXR
F/FLDLR
−/− mice by 65% and 77%, respectively. By contrast, DCHP did not affect atherosclerosis development in PXR
ΔMyeLDLR
−/− mice. Exposure to DCHP led to elevated expression of the scavenger receptor CD36 in macrophages and increased macrophage form cell formation in PXR
F/FLDLR
−/− mice. Our findings provide potential mechanisms underlying phthalate-associated CVD risk and will ultimately stimulate further investigations and mitigation of the adverse effects of plastic-associated EDCs on CVD risk in humans.
Full article
►▼
Show Figures
Open AccessReview
Nuclear Receptor PXR in Drug-Induced Hypercholesterolemia
by
Mikko Karpale, Janne Hukkanen and Jukka Hakkola
Cited by 15 | Viewed by 4364
Abstract
Atherosclerosis is a major global health concern. The central modifiable risk factors and causative agents of the disease are high total and low-density lipoprotein (LDL) cholesterol. To reduce morbidity and mortality, a thorough understanding of the factors that influence an individual’s cholesterol status
[...] Read more.
Atherosclerosis is a major global health concern. The central modifiable risk factors and causative agents of the disease are high total and low-density lipoprotein (LDL) cholesterol. To reduce morbidity and mortality, a thorough understanding of the factors that influence an individual’s cholesterol status during the decades when the arteria-narrowing arteriosclerotic plaques are forming is critical. Several drugs are known to increase cholesterol levels; however, the mechanisms are poorly understood. Activation of pregnane X receptor (PXR), the major regulator of drug metabolism and molecular mediator of clinically significant drug–drug interactions, has been shown to induce hypercholesterolemia. As a major sensor of the chemical environment, PXR may in part mediate hypercholesterolemic effects of drug treatment. This review compiles the current knowledge of PXR in cholesterol homeostasis and discusses the role of PXR in drug-induced hypercholesterolemia.
Full article
►▼
Show Figures
Open AccessArticle
Phosphorylation-Induced Ubiquitination and Degradation of PXR through CDK2-TRIM21 Axis
by
Mengyao Qin, Yu Xin, Yong Bian, Xuan Yang, Tao Xi and Jing Xiong
Cited by 11 | Viewed by 4147
Abstract
Pregnane X receptor (PXR) is a member of the nuclear receptor superfamily that is activated by a variety of endogenous metabolites or xenobiotics. Its downstream target genes are involved in metabolism, inflammation and processes closely related to cancer. However, the stability regulation of
[...] Read more.
Pregnane X receptor (PXR) is a member of the nuclear receptor superfamily that is activated by a variety of endogenous metabolites or xenobiotics. Its downstream target genes are involved in metabolism, inflammation and processes closely related to cancer. However, the stability regulation of PXR protein resulting from post-translational modification is still largely undefined. In the present study, primary mouse hepatocytes, hepatoma HepG2 cells and HEK 293T cells were used to investigate gene expression and protein interactions. The role of kinases was evaluated by RNA interference and overexpression constructs with or without PXR phosphorylation site mutations. The activity of CYP3A4 and P-gp was determined by enzymatic and substrate accumulation assays. It was found that E3 ubiquitin ligase TRIM21 mediates the ubiquitination and degradation of PXR and plays an important role in regulating the activity of PXR. On this basis, PXR phosphorylation-associated kinases were evaluated regarding regulation of the stability of PXR. We found cyclin dependent kinase 2 (CDK2) exclusively phosphorylates PXR at Ser350, promotes its disassociation with Hsp90/DNAJC7, and leads to subsequent TRIM21-mediated PXR ubiquitination and degradation. As well-known CDK inhibitors, dinaciclib and kenpaullone stabilize PXR and result in elevated expression and activity of PXR-targeted DMETs, including carboxylesterases, CYP3A4 and P-gp. The suppressed degradation of PXR by CDK2 inhibitors denotes dinaciclib-induced promotion of PXR-targeted genes. The findings of CDK2-mediated PXR degradation indicate a wide range of potential drug–drug interactions during clinical cancer therapy using CDK inhibitors and imply an alternative direction for the development of novel PXR antagonists.
Full article
►▼
Show Figures
Open AccessReview
The Nuclear Receptor PXR in Chronic Liver Disease
by
Katia Sayaf, Ilaria Zanotto, Francesco Paolo Russo, Daniela Gabbia and Sara De Martin
Cited by 22 | Viewed by 4672
Abstract
Pregnane X receptor (PXR), a nuclear receptor known for modulating the transcription of drug metabolizing enzymes and transporters (DMETs), such as cytochrome P450 3A4 and P-glycoprotein, is functionally involved in chronic liver diseases of different etiologies. Furthermore, PXR activity relates to that of
[...] Read more.
Pregnane X receptor (PXR), a nuclear receptor known for modulating the transcription of drug metabolizing enzymes and transporters (DMETs), such as cytochrome P450 3A4 and P-glycoprotein, is functionally involved in chronic liver diseases of different etiologies. Furthermore, PXR activity relates to that of other NRs, such as constitutive androstane receptor (CAR), through a crosstalk that in turn orchestrates a complex network of responses. Thus, besides regulating DMETs, PXR signaling is involved in both liver damage progression and repair and in the neoplastic transition to hepatocellular carcinoma. We here summarize the present knowledge about PXR expression and function in chronic liver diseases characterized by different etiologies and clinical outcome, focusing on the molecular pathways involved in PXR activity. Although many molecular details of these finely tuned networks still need to be fully understood, we conclude that PXR and its modulation could represent a promising pharmacological target for the identification of novel therapeutical approaches to chronic liver diseases.
Full article
►▼
Show Figures
Open AccessArticle
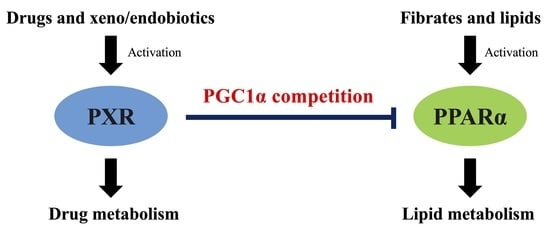
PXR Suppresses PPARα-Dependent HMGCS2 Gene Transcription by Inhibiting the Interaction between PPARα and PGC1α
by
Ryota Shizu, Kanako Ezaki, Takumi Sato, Ayaka Sugawara, Takuomi Hosaka, Takamitsu Sasaki and Kouichi Yoshinari
Cited by 9 | Viewed by 3271
Abstract
Background: PXR is a xenobiotic-responsive nuclear receptor that controls the expression of drug-metabolizing enzymes. Drug-induced activation of PXR sometimes causes drug–drug interactions due to the induced metabolism of co-administered drugs. Our group recently reported a possible drug–drug interaction mechanism via an interaction between
[...] Read more.
Background: PXR is a xenobiotic-responsive nuclear receptor that controls the expression of drug-metabolizing enzymes. Drug-induced activation of PXR sometimes causes drug–drug interactions due to the induced metabolism of co-administered drugs. Our group recently reported a possible drug–drug interaction mechanism via an interaction between the nuclear receptors CAR and PPARα. As CAR and PXR are structurally and functionally related receptors, we investigated possible crosstalk between PXR and PPARα. Methods: Human hepatocyte-like HepaRG cells were treated with various PXR ligands, and mRNA levels were determined by quantitative reverse transcription PCR. Reporter assays using the
HMGCS2 promoter containing a PPARα-binding motif and mammalian two-hybrid assays were performed in HepG2 or COS-1 cells. Results: Treatment with PXR activators reduced the mRNA levels of PPARα target genes in HepaRG cells. In reporter assays, PXR suppressed PPARα-dependent gene expression in HepG2 cells. In COS-1 cells, co-expression of PGC1α, a common coactivator of PPARα and PXR, enhanced PPARα-dependent gene transcription, which was clearly suppressed by PXR. Consistently, in mammalian two-hybrid assays, the interaction between PGC1α and PPARα was attenuated by ligand-activated PXR. Conclusion: The present results suggest that ligand-activated PXR suppresses PPARα-dependent gene expression by inhibiting PGC1α recruitment.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
Phenobarbital Induces SLC13A5 Expression through Activation of PXR but Not CAR in Human Primary Hepatocytes
by
Zhihui Li, Linhao Li, Scott Heyward, Shuaiqian Men, Meishu Xu, Tatsuya Sueyoshi and Hongbing Wang
Cited by 6 | Viewed by 2862
Abstract
Phenobarbital (PB), a widely used antiepileptic drug, is known to upregulate the expression of numerous drug-metabolizing enzymes and transporters in the liver primarily via activation of the constitutive androstane receptor (CAR, NR1I3). The solute carrier family 13 member 5 (SLC13A5), a sodium-coupled citrate
[...] Read more.
Phenobarbital (PB), a widely used antiepileptic drug, is known to upregulate the expression of numerous drug-metabolizing enzymes and transporters in the liver primarily via activation of the constitutive androstane receptor (CAR, NR1I3). The solute carrier family 13 member 5 (SLC13A5), a sodium-coupled citrate transporter, plays an important role in intracellular citrate homeostasis that is associated with a number of metabolic syndromes and neurological disorders. Here, we show that PB markedly elevates the expression of SLC13A5 through a pregnane X receptor (PXR)-dependent but CAR-independent signaling pathway. In human primary hepatocytes, the mRNA and protein expression of SLC13A5 was robustly induced by PB treatment, while genetic knockdown or pharmacological inhibition of PXR significantly attenuated this induction. Utilizing genetically modified HepaRG cells, we found that PB induces SLC13A5 expression in both wild type and CAR-knockout HepaRG cells, whereas such induction was fully abolished in the PXR-knockout HepaRG cells. Mechanistically, we identified and functionally characterized three enhancer modules located upstream from the transcription start site or introns of the
SLC13A5 gene that are associated with the regulation of PXR-mediated SLC13A5 induction. Moreover, metformin, a deactivator of PXR, dramatically suppressed PB-mediated induction of hepatic SLC13A5 as well as its activation of the SLC13A5 luciferase reporter activity via PXR. Collectively, these data reveal PB as a potent inducer of SLC13A5 through the activation of PXR but not CAR in human primary hepatocytes.
Full article
►▼
Show Figures
Open AccessReview
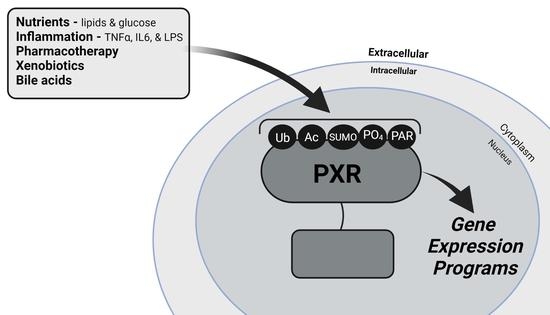
The Interface between Cell Signaling Pathways and Pregnane X Receptor
by
Robert S. Rogers, Annemarie Parker, Phill D. Vainer, Elijah Elliott, Dakota Sudbeck, Kaushal Parimi, Venkata P. Peddada, Parker G. Howe, Nick D’Ambrosio, Gregory Ruddy, Kaitlin Stackable, Megan Carney, Lauren Martin, Thomas Osterholt and Jeff L. Staudinger
Cited by 9 | Viewed by 4389
Abstract
Highly expressed in the enterohepatic system, pregnane X receptor (PXR, NR1I2) is a well-characterized nuclear receptor (NR) that regulates the expression of genes in the liver and intestines that encode key drug metabolizing enzymes and drug transporter proteins in mammals. The net effect
[...] Read more.
Highly expressed in the enterohepatic system, pregnane X receptor (PXR, NR1I2) is a well-characterized nuclear receptor (NR) that regulates the expression of genes in the liver and intestines that encode key drug metabolizing enzymes and drug transporter proteins in mammals. The net effect of PXR activation is to increase metabolism and clear drugs and xenobiotics from the body, producing a protective effect and mediating clinically significant drug interaction in patients on combination therapy. The complete understanding of PXR biology is thus important for the development of safe and effective therapeutic strategies. Furthermore, PXR activation is now known to specifically transrepress the inflammatory- and nutrient-signaling pathways of gene expression, thereby providing a mechanism for linking these signaling pathways together with enzymatic drug biotransformation pathways in the liver and intestines. Recent research efforts highlight numerous post-translational modifications (PTMs) which significantly influence the biological function of PXR. However, this thrust of research is still in its infancy. In the context of gene-environment interactions, we present a review of the recent literature that implicates PXR PTMs in regulating its clinically relevant biology. We also provide a discussion of how these PTMs likely interface with each other to respond to extracellular cues to appropriately modify PXR activity.
Full article
►▼
Show Figures
Open AccessReview
Regulation of PXR Function by Coactivator and Corepressor Proteins: Ligand Binding Is Just the Beginning
by
Juan Pablo Rigalli, Dirk Theile, Julie Nilles and Johanna Weiss
Cited by 9 | Viewed by 5043
Abstract
The pregnane X receptor (PXR,
NR1I2) is a nuclear receptor which exerts its regulatory function by heterodimerization with the retinoid-X-receptor α (RXRα,
NR2B1) and binding to the promoter and enhancer regions of diverse target genes. PXR is involved in the regulation
[...] Read more.
The pregnane X receptor (PXR,
NR1I2) is a nuclear receptor which exerts its regulatory function by heterodimerization with the retinoid-X-receptor α (RXRα,
NR2B1) and binding to the promoter and enhancer regions of diverse target genes. PXR is involved in the regulation of drug metabolism and excretion, metabolic and immunological functions and cancer pathogenesis. PXR activity is strongly regulated by the association with coactivator and corepressor proteins. Coactivator proteins exhibit histone acetyltransferase or histone methyltransferase activity or associate with proteins having one of these activities, thus promoting chromatin decondensation and activation of the gene expression. On the contrary, corepressor proteins promote histone deacetylation and therefore favor chromatin condensation and repression of the gene expression. Several studies pointed to clear cell- and ligand-specific differences in the activation of PXR. In this article, we will review the critical role of coactivator and corepressor proteins as molecular determinants of the specificity of PXR-mediated effects. As already known for other nuclear receptors, understanding the complex mechanism of PXR activation in each cell type and under particular physiological and pathophysiological conditions may lead to the development of selective modulators with therapeutic potential.
Full article
►▼
Show Figures