Journal Description
Organoids
Organoids
is an international, peer-reviewed, open access journal on all aspects of organoids published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 19.3 days after submission; acceptance to publication is undertaken in 2.7 days (median values for papers published in this journal in the second half of 2024).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
- Organoids is a companion journal of Cells.
Latest Articles
Precision Medicine for Peritoneal Carcinomatosis—Current Advances in Organoid Drug Testing and Clinical Applicability
Organoids 2025, 4(1), 2; https://doi.org/10.3390/organoids4010002 - 24 Jan 2025
Abstract
Peritoneal carcinomatosis from gastrointestinal tumours is considered a poor prognostic factor, with a median overall survival of six to nine months in the absence of intervention. The advent of patient-derived organoid cultures (PDOs) has provided a breakthrough in personalised medicine, allowing researchers and
[...] Read more.
Peritoneal carcinomatosis from gastrointestinal tumours is considered a poor prognostic factor, with a median overall survival of six to nine months in the absence of intervention. The advent of patient-derived organoid cultures (PDOs) has provided a breakthrough in personalised medicine, allowing researchers and clinicians to model the complexity and heterogeneity of individual tumours in vitro. PDOs hold great promise in this field, as variations in the management of peritoneal carcinomatosis due to differences in the method of delivery of chemotherapeutics, drug selection, exposure duration, and tumour pathology make it impractical to use a single, standardised treatment regimen. We aim to summarise the methodologies and limitations of studies encapsulating organoids derived from peritoneal metastases to encourage design considerations that may improve future clinical relevance, standardise protocols, and address translational challenges in personalising treatment strategies.
Full article
(This article belongs to the Special Issue Advances in Organoid Technology: Bridging the Gap between Research and Therapy)
►
Show Figures
Open AccessArticle
Three-Dimensional Morphological Characterisation of Human Cortical Organoids Using a Customised Image Analysis Workflow
by
Sarah Handcock, Kay Richards, Timothy J. Karle, Pamela Kairath, Alita Soch, Carolina A. Chavez, Steven Petrou and Snezana Maljevic
Organoids 2025, 4(1), 1; https://doi.org/10.3390/organoids4010001 - 17 Jan 2025
Abstract
►▼
Show Figures
Summary Statement: A tailored image analysis workflow was applied to quantify cortical organoid health, development, morphology and cellular composition over time. The assessment of cellular composition and viability of stem cell-derived organoid models is a complex but essential approach to understanding the
[...] Read more.
Summary Statement: A tailored image analysis workflow was applied to quantify cortical organoid health, development, morphology and cellular composition over time. The assessment of cellular composition and viability of stem cell-derived organoid models is a complex but essential approach to understanding the mechanisms of human development and disease. Aim: Our study was motivated by the need for an image-analysis workflow, including high-cell content, high-throughput methods, to measure the architectural features of developing organoids. We assessed stem cell-derived cortical organoids at 4 and 6 months post-induction using immunohistochemistry-labelled sections as the analysis testbed. The workflow leveraged fluorescence imaging tailored to classify cells as viable and dying or non-viable and assign neuronal and astrocytic perinuclear markers to count cells. Results/Outcomes: Image acquisition was accelerated by capturing the organoid slice in 3D using widefield-fluorescence microscopy. This method used computational clearing to resolve nuclear and perinuclear markers and retain their spatial information within the organoid’s heterogeneous structure. The customised workflow analysed over 1.5 million cells using DAPI-stained nuclei, filtering and quantifying viable and non-viable cells and the necrotic-core regions. Temporal analyses of neuronal cell number derived from perinuclear labelling were consistent with organoid maturation from 4 to 6 months of in vitro differentiation. Overall: We have provided a comprehensive and enhanced image analysis workflow for organoid structural evaluation, creating the ability to gather cellular-level statistics in control and disease models.
Full article

Figure 1
Open AccessArticle
Pre-Adipocytes in 3D Co-Culture Underwent Self-Differentiation: New Perspectives for an Old Model
by
Tamara Dal-Mora, Najla Adel Saleh, Veridiana Pacheco Goulart Martinazzo, Maria Luiza Carneiro Buchele, Michele Patrícia Rode, Adny Henrique Silva, Laura Sartori Assunção, Tânia Beatriz Creczynski-Pasa and Fabiola Branco Filippin-Monteiro
Organoids 2024, 3(4), 295-308; https://doi.org/10.3390/organoids3040018 - 18 Dec 2024
Abstract
►▼
Show Figures
Adipogenesis is a complex process influenced by various cellular interactions within adipose tissue, which plays a critical role in metabolic homeostasis. This study aimed to develop a novel in vitro three-dimensional (3D) co-culture model using murine 3T3-L1 pre-adipocytes, J774 macrophages, and NIH-3T3 fibroblasts
[...] Read more.
Adipogenesis is a complex process influenced by various cellular interactions within adipose tissue, which plays a critical role in metabolic homeostasis. This study aimed to develop a novel in vitro three-dimensional (3D) co-culture model using murine 3T3-L1 pre-adipocytes, J774 macrophages, and NIH-3T3 fibroblasts to investigate adipogenic differentiation and inflammatory pathways. We first validated an adipogenic differentiation protocol in a two-dimensional (2D) model, where 3T3-L1 pre-adipocytes were subjected to a hormonal medium containing 3-isobutyl-1-methylxanthine, dexamethasone and insulin. After 7 days, differentiated cells were analyzed using Oil Red O and Nile Red staining, confirming lipid accumulation. Subsequently, spheroids were formed in 3D cultures, with monospheroids and heterospheroids maintained in either control medium or MDI for 11 days. Size measurements indicated significant growth in heterospheroids, particularly in the 3T3-L1:J774 combination, underscoring the importance of cellular interactions. Confocal microscopy and flow cytometry analyses demonstrated that even in the absence of hormonal stimuli, control spheroids exhibited adipogenic differentiation, evidenced by a notable proportion of Nile Red-positive cells (75.7 ± 1.7%). Inflammatory profiling revealed that the heterospheroid 3:J produced the highest levels of nitric oxide (NO), with no significant differences observed between control and MDI conditions. This study highlights the potential of 3D co-culture systems for elucidating the intricate interactions among adipocytes, macrophages, and fibroblasts. The findings may provide valuable insights into novel therapeutic targets for metabolic disorders.
Full article

Figure 1
Open AccessFeature PaperArticle
Establishment and Validation of Patient-Derived Non-Small Cell Lung Cancer Organoids as In Vitro Lung Cancer Models
by
Raphael S. Werner, Jae-Hwi Jang, Markus Rechsteiner, Michaela B. Kirschner and Isabelle Opitz
Organoids 2024, 3(4), 281-294; https://doi.org/10.3390/organoids3040017 - 9 Nov 2024
Abstract
►▼
Show Figures
Background: Recent advances in the personalized treatment of non-small cell lung cancer (NSCLC) require representative in vitro model systems that reflect tumor heterogeneity and maintain the characteristic genetic aberrations. We therefore aimed to establish patient-derived NSCLC organoids that offer a reliable platform for
[...] Read more.
Background: Recent advances in the personalized treatment of non-small cell lung cancer (NSCLC) require representative in vitro model systems that reflect tumor heterogeneity and maintain the characteristic genetic aberrations. We therefore aimed to establish patient-derived NSCLC organoids that offer a reliable platform for further investigations. Methods: NSCLC organoids were cultured between May 2020 and February 2022 from surgically resected NSCLC tissue specimens. After histological and immunohistochemical validation, genetic validation was performed by targeted next-generation sequencing of tissue and organoid specimens using the Oncomine Focus Assay (ThermoFisher Scientific). Results: From 37 resected NSCLC samples, 18 primary organoid cultures were successfully established and expanded during early passages. Upon histomorphological validation, organoids showed complementary characteristics when compared to the resected parental tumor, including adenocarcinoma, squamous cell carcinoma, mucoepidermoid carcinoma, and lung carcinoid differentiation. Among nine parental tumors, traceable genetic alterations were detected, and three corresponding organoids lines retained this mutational profile, including a KRAS p.Gly12Val mutation, KRAS p.Gly12Cys mutation, and RET-fusion. Conclusions: The establishment of primary NSCLC organoids from surgically resected tissue is feasible. Histological, immunohistochemical, and genetic validation is essential to identify representative NSCLC organoids that maintain the characteristics of the parental tumor. Overall, low establishment rates remain a challenge for broad clinical applications.
Full article

Figure 1
Open AccessReview
Precision Medicine for Gastric Cancer: Current State of Organoid Drug Testing
by
Tharindie N. Silva, Josephine A. Wright, Daniel L. Worthley and Susan L. Woods
Organoids 2024, 3(4), 266-280; https://doi.org/10.3390/organoids3040016 - 31 Oct 2024
Abstract
Gastric cancer (GC) presents a significant health challenge and ranks as the fifth most common cancer in the world. Unfortunately, most patients with GC exhaust standard care treatment options due to late diagnosis and tumour heterogeneity that leads to drug resistance, resulting in
[...] Read more.
Gastric cancer (GC) presents a significant health challenge and ranks as the fifth most common cancer in the world. Unfortunately, most patients with GC exhaust standard care treatment options due to late diagnosis and tumour heterogeneity that leads to drug resistance, resulting in poor survival outcomes. Potentially, this situation can be improved by personalising treatment choice. Organoids are an emerging cell model system that recapitulates tumour heterogeneity and drug responses. Coupled with genomic analysis, organoid culture can be used to guide personalised medicine. The GC organoid field, however, lacks standardised methodologies for assessing organoid drug sensitivities. Comparing results across different GC organoid studies and correlating organoid drug responses with patient outcomes is challenging. Hence, we aim to summarise the methodologies used in GC organoid drug testing and correlation with clinical outcomes and discuss design considerations and limitations to enhance the robustness of such studies in the future.
Full article
(This article belongs to the Special Issue Advances in Organoid Technology: Bridging the Gap between Research and Therapy)
►▼
Show Figures

Figure 1
Open AccessReview
Bioengineering Tooth and Periodontal Organoids from Stem and Progenitor Cells
by
Fuad Gandhi Torizal, Syarifah Tiara Noorintan and Zakiya Gania
Organoids 2024, 3(4), 247-265; https://doi.org/10.3390/organoids3040015 - 3 Oct 2024
Cited by 1
Abstract
►▼
Show Figures
Tooth and periodontal organoids from stem and progenitor cells represent a significant advancement in regenerative dentistry, offering solutions for tooth loss and periodontal diseases. These organoids, which mimic the architecture and function of real organs, provide a cutting-edge platform for studying dental biology
[...] Read more.
Tooth and periodontal organoids from stem and progenitor cells represent a significant advancement in regenerative dentistry, offering solutions for tooth loss and periodontal diseases. These organoids, which mimic the architecture and function of real organs, provide a cutting-edge platform for studying dental biology and developing therapies. Recent methodologies have been developed to optimize conditions for organoid production, advancing dental regenerative medicine, disease modeling, and developmental studies. The integration of bioengineering strategies with culture techniques enhances both our understanding and the therapeutic potential of these organoids. Additionally, factors such as the extracellular matrix, growth factors, and culture systems profoundly influence organoid formation and maturation. This review explores various bioengineering approaches for generating organoids, emphasizing the pivotal role of stem and progenitor cells.
Full article

Figure 1
Open AccessReview
Recent Advances and Future Perspectives in Vascular Organoids and Vessel-on-Chip
by
Gowtham Reddy Cheruku, Chloe Veronica Wilson, Suriya Raviendran and Qingzhong Xiao
Organoids 2024, 3(3), 203-246; https://doi.org/10.3390/organoids3030014 - 4 Sep 2024
Abstract
►▼
Show Figures
Recent advancements in vascular organoid (VO) and vessel-on-chip (VoC) technologies have revolutionized our approach to studying human diseases, offering unprecedented insights through more physiologically relevant models. VOs generated from human pluripotent stem cells exhibit remarkable self-organization capabilities, forming complex three-dimensional structures that closely
[...] Read more.
Recent advancements in vascular organoid (VO) and vessel-on-chip (VoC) technologies have revolutionized our approach to studying human diseases, offering unprecedented insights through more physiologically relevant models. VOs generated from human pluripotent stem cells exhibit remarkable self-organization capabilities, forming complex three-dimensional structures that closely mimic human blood vessel architecture and function, while VoCs are engineered with microfluidic systems that meticulously recreate the physical and functional attributes of blood vessels. These innovative constructs serve as powerful tools for investigating vascular development, disease progression, and therapeutic efficacy. By enabling the creation of patient-specific VOs and VoCs, they pave the way for personalized medicine approaches, allowing researchers to delve into genetic variations, intricate cellular interactions, and dynamic processes with exceptional resolution. The synergy between VOs and VoCs with newly developed cutting-edge technologies has further amplified their potential, unveiling novel mechanisms underlying human pathologies and identifying promising therapeutic targets. Herein, we summarize different types of VOs and VoCs and present an extensive overview on the generation and applications of VOs and VoCs. We will also highlight clinical and translational challenges and future perspectives around VOs and VoCs.
Full article

Figure 1
Open AccessStudy Protocol
A Method to Study Migration and Invasion of Mouse Intestinal Organoids
by
Valérie M. Wouters, Ciro Longobardi and Jan Paul Medema
Organoids 2024, 3(3), 194-202; https://doi.org/10.3390/organoids3030013 - 21 Aug 2024
Abstract
Colorectal cancer (CRC) is the third most common cancer worldwide and it is the second leading cause of cancer death. In CRC, as in most cancers, the formation of metastasis through the migration and invasion of cancer cells to distant organs is associated
[...] Read more.
Colorectal cancer (CRC) is the third most common cancer worldwide and it is the second leading cause of cancer death. In CRC, as in most cancers, the formation of metastasis through the migration and invasion of cancer cells to distant organs is associated with a dismal prognosis. The study of the mechanisms associated with cancer, and, in particular, CRC, changed in the last decade due to the introduction of organoids. These represent a step forward in terms of complexity from cell lines and allowed the use of mouse models in cancer research to be limited. Although organoids faithfully model the cellular complexity of CRC, current protocols do not allow for the use of organoids in some crucial processes of metastasis, such as migration and invasion. In this study, a method to study migration and invasion using mouse intestinal organoids in vitro is presented. This protocol provides researchers with the opportunity to investigate the migratory behavior of organoid lines and study the impact of distinct mutations on the migratory and invasive capacity of cancer cells.
Full article
(This article belongs to the Special Issue Organoids and Cancer Models)
►▼
Show Figures

Figure 1
Open AccessReview
Trophoblast Organoids: Capturing the Complexity of Early Placental Development In Vitro
by
Brady M. Wessel, Jenna N. Castro and Victoria H. J. Roberts
Organoids 2024, 3(3), 174-193; https://doi.org/10.3390/organoids3030012 - 2 Aug 2024
Abstract
►▼
Show Figures
First trimester placental development comprises some of the most critical yet understudied events that impact fetal development. Improper placentation leads to a host of health issues that not only impact the fetal period but also influence offspring throughout their lives. Thus, a paradigm
[...] Read more.
First trimester placental development comprises some of the most critical yet understudied events that impact fetal development. Improper placentation leads to a host of health issues that not only impact the fetal period but also influence offspring throughout their lives. Thus, a paradigm to study early placental development is necessary, and this has spurred on the pursuit of new in vitro model systems that recapitulate specific aspects of placentation. One of the most complex and translationally valid models to arise are organoids, three-dimensional structures comprising multiple differentiated cell types that originate from a common progenitor population. Trophoblasts are the progenitor cells of the placenta, serving as the proliferative base for placental development. Recent advances have enabled the derivation of organoids from primary tissue, yet access to first trimester human samples is ethically constrained; derivation from established trophoblast stem cell lines is an alternative source. Organoids have already proven useful in generating insights into molecular events that underlie trophoblast differentiation, with the identification of new cell subtypes that are primed to differentiate down different paths. In this review, (1) we recap early pregnancy development events, (2) provide an overview of the cellular complexity of the placenta, (3) discuss the generation of organoids from tissue versus cellular sources, (4) highlight the value of translational animal models, and (5) focus on the complexities of the molecular regulation of trophoblast organoid development, differentiation, and function.
Full article

Graphical abstract
Open AccessOpinion
Organoids and 3D In Vitro Models as a Platform for Precision Medicine (PM): An Update
by
Payal Ganguly
Organoids 2024, 3(3), 165-173; https://doi.org/10.3390/organoids3030011 - 1 Aug 2024
Abstract
►▼
Show Figures
Globally, a number of diseases impact us and while treatment options exist, it is often found that similar treatments have variable effects on different patients with the same disease. Particularly in the case of conditions that are closely associated with genetics (like cancer),
[...] Read more.
Globally, a number of diseases impact us and while treatment options exist, it is often found that similar treatments have variable effects on different patients with the same disease. Particularly in the case of conditions that are closely associated with genetics (like cancer), the intensity and results of a treatment vary between patients. Even for diseases like arthritis it is not uncommon for only a fraction of patients to achieve remission with the same therapeutic approach. With millions suffering from diseases like cancer and arthritis, precision medicine (PM) has been at the forefront of biomedical and pharmaceutical research since 2015. PM focusses on understanding the genetic and environmental factors affecting the patients and has several platforms. One of the platforms is the use of three-dimensional (3D) in vitro models, especially those derived from the patient themselves. These models, like organ-on-chip (OOC), organoid and spheroid models, 3D biomaterial scaffolds and others, have several advantages over traditional two-dimensional (2D) cell culture approaches. In this opinion paper, the author briefly discusses the different platforms used for PM. Then, the advantages that 3D in vitro models have over traditional 2D models and in vivo models are considered and an overview of their applications is provided. Finally, the author outlines the challenges and future directions and shares their opinion about using 3D in vitro models as a tool for PM towards enhanced patient outcomes.
Full article

Figure 1
Open AccessArticle
Heparin-Binding Epidermal-like Growth Factor (HB-EGF) Reduces Cell Death in an Organoid Model of Retinal Damage
by
Michelle N. H. Tang, Mariya Moosajee, Najam A. Sharif, G. Astrid Limb and Karen Eastlake
Organoids 2024, 3(3), 148-164; https://doi.org/10.3390/organoids3030010 - 5 Jul 2024
Abstract
In zebrafish and various mammalian species, HB-EGF has been shown to promote Müller glia proliferation and activation of repair mechanisms that have not been fully investigated in human retina. In the current study, 70- to 90-day-old human retinal organoids were treated with 20
[...] Read more.
In zebrafish and various mammalian species, HB-EGF has been shown to promote Müller glia proliferation and activation of repair mechanisms that have not been fully investigated in human retina. In the current study, 70- to 90-day-old human retinal organoids were treated with 20 μM 4-hydroxytamoxifen (4-OHT), and CRX, REC, NRL, PAX6, VIM, GFAP, and VSX2 gene and protein expression were assessed at various times points after treatment. Organoids with or without 4-OHT-induced damage were then cultured with HB-EGF for 7 days. We showed that 20 μM 4-OHT caused a reduction in the number of recoverin-positive cells; an increase in the number of TUNEL-positive cells; and downregulation of the photoreceptor gene markers CRX, NRL, and REC. Culture of organoids with HB-EGF for 7 days after 4-OHT-induced damage caused a marked reduction in the number of TUNEL-positive cells and small increases in the number of Ki67-positive cells and PAX6 and NOTCH1 gene expression. The current results suggest that treatment of human ESC-derived retinal organoids with 4-OHT may be used as a model of retinal degeneration in vitro. Furthermore, HB-EGF treatment of human retinal organoids increases proliferating Müller cells, but only after 4-OHT induced damage, and may be an indication of Muller reactivity in response to photoreceptor damage. Further studies will aim to identify factors that may induce Müller cell-mediated regeneration of the human retina, aiding in the development of therapies for retinal degeneration.
Full article
(This article belongs to the Special Issue The Current Applications and Potential of Stem Cell-Derived Organoids)
►▼
Show Figures

Figure 1
Open AccessArticle
Single-Cell Assessment of Human Stem Cell-Derived Mesolimbic Models and Their Responses to Substances of Abuse
by
Thomas P. Rudibaugh, Ryan W. Tam, R. Chris Estridge, Samantha R. Stuppy and Albert J. Keung
Organoids 2024, 3(2), 126-147; https://doi.org/10.3390/organoids3020009 - 20 Jun 2024
Abstract
The mesolimbic pathway connects ventral tegmental area dopaminergic neurons and striatal medium spiny neurons, playing a critical role in reward and stress behaviors. Exposure to substances of abuse during development and adulthood has been linked to adverse outcomes and molecular changes. The rise
[...] Read more.
The mesolimbic pathway connects ventral tegmental area dopaminergic neurons and striatal medium spiny neurons, playing a critical role in reward and stress behaviors. Exposure to substances of abuse during development and adulthood has been linked to adverse outcomes and molecular changes. The rise of human cell repositories and whole-genome sequences enables human functional genomics ‘in a dish’, offering insights into human-specific responses to substances of abuse. Continued development of new models is needed, and the characterization of in vitro models is also necessary to ensure appropriate experimental designs and the accurate interpretation of results. This study introduces new culture conditions for generating medium spiny neurons and dopaminergic neurons with an early common media, allowing for coculture and assembloid generation. It then provides a comprehensive characterization of these and prior models and their responses to substances of abuse. Single-cell analysis reveals cell-type-specific transcriptomic responses to dopamine, cocaine, and morphine, including compound and cell-type-specific transcriptomic signatures related to neuroinflammation and alterations in signaling pathways. These findings offer a resource for future genomics studies leveraging human stem cell-derived models.
Full article
(This article belongs to the Special Issue The Current Applications and Potential of Stem Cell-Derived Organoids)
►▼
Show Figures

Figure 1
Open AccessProtocol
Development and Optimization of a Lactate Dehydrogenase Assay Adapted to 3D Cell Cultures
by
Héloïse Castiglione, Lucie Madrange, Thomas Lemonnier, Jean-Philippe Deslys, Frank Yates and Pierre-Antoine Vigneron
Organoids 2024, 3(2), 113-125; https://doi.org/10.3390/organoids3020008 - 5 Jun 2024
Abstract
►▼
Show Figures
In recent years, 3D cell culture systems have emerged as sophisticated in vitro models, providing valuable insights into human physiology and diseases. The transition from traditional 2D to advanced 3D cultures has introduced novel obstacles, complicating the characterization and analysis of these models.
[...] Read more.
In recent years, 3D cell culture systems have emerged as sophisticated in vitro models, providing valuable insights into human physiology and diseases. The transition from traditional 2D to advanced 3D cultures has introduced novel obstacles, complicating the characterization and analysis of these models. While the lactate dehydrogenase (LDH) activity assay has long been a standard readout for viability and cytotoxicity assessments in 2D cultures, its applicability in long-term 3D cultures is hindered by inappropriate normalization and low LDH stability over time. In response to these challenges, we propose an optimization of LDH assays, including a crucial normalization step based on total protein quantification and a storage method using an LDH preservation buffer. We applied it to compare unexposed cerebral organoids with organoids exposed to a toxic dose of valproic acid, and showed efficient normalization of cellular viability as well as enhanced LDH stability within the buffer. Importantly, normalized LDH activity results obtained were independent of organoid dimension and cell density. This refined LDH assay, tailored to address 3D culture constraints, allows for the transposition of this routine test from 2D to 3D cultures.
Full article

Figure 1
Open AccessCommunication
Organoids, Biocybersecurity, and Cyberbiosecurity—A Light Exploration
by
Xavier Palmer, Cyril Akafia, Eleasa Woodson, Amanda Woodson and Lucas Potter
Organoids 2024, 3(2), 83-112; https://doi.org/10.3390/organoids3020007 - 13 May 2024
Abstract
►▼
Show Figures
Organoids present immense promise for studying organ systems and their functionality. Recently, they have become the subject of exploration outside of purely biomedical uses in multiple directions. We will explore the rapidly evolving landscape of organoid research over the 21st century, discussing significant
[...] Read more.
Organoids present immense promise for studying organ systems and their functionality. Recently, they have become the subject of exploration outside of purely biomedical uses in multiple directions. We will explore the rapidly evolving landscape of organoid research over the 21st century, discussing significant advancements in organoid research and highlighting breakthroughs, methodologies, and their transformative impact on our understanding of physiology and modeling. In addition, we will explore their potential use for biocomputing and harnessing organoid intelligence, investigate how these miniaturized organ-like structures promise to create novel computational models and processing platforms allowing for innovative approaches in drug discovery, personalized medicine, and disease prediction. Lastly, we will address the ethical dilemmas surrounding organoid research by dissecting the intricate ethical considerations related to the creation, use, and potential implications of these in vitro models. Through this work, the goal of this paper is to provide introductory perspectives and bridges that will connect organoids to cybersecurity applications and the imperative ethical discourse accompanying its advancements with commentary on future uses.
Full article

Figure 1
Open AccessReview
Treatment of Canine Type 1 Diabetes Mellitus: The Long Road from Twice Daily Insulin Injection towards Long-Lasting Cell-Based Therapy
by
Flavia C. M. Oliveira, Annemarie W. Y. Voorbij, Elisa C. Pereira, Leonor M. M. Alves e Almeida, Geanne R. Moraes, Joana T. De Oliveira, Boyd H. T. Gouw, Sabrina A. M. Legatti, Hans S. Kooistra, Bart Spee, Andre M. C. Meneses and Louis C. Penning
Organoids 2024, 3(2), 67-82; https://doi.org/10.3390/organoids3020006 - 4 Apr 2024
Cited by 1
Abstract
►▼
Show Figures
For over 150 years, researchers have studied the (patho)physiology of the endocrine pancreas and devised treatment options for diabetes mellitus (DM). However, no cure has been developed so far. In dogs, diabetes mellitus type 1 (T1DM) is the most common presentation. Treatment consists
[...] Read more.
For over 150 years, researchers have studied the (patho)physiology of the endocrine pancreas and devised treatment options for diabetes mellitus (DM). However, no cure has been developed so far. In dogs, diabetes mellitus type 1 (T1DM) is the most common presentation. Treatment consists of twice daily insulin injections, monitored by spatial blood glucose measurements. Even though dogs were instrumental in the discovery of insulin and islet transplantations, the treatment in diabetic dogs has remained unchanged for decades. Providing twice daily insulin injections is demanding for both owners and dogs and may result in hypoglycaemic events, creating the need for new treatment strategies. Novel regenerative medicine-based tools, such as improved β-cell culture protocols and artificial devices, have sparked hope for a cure. In human medicine, emerging technologies such as the transplantation of insulin-producing β-cells, generated by stem cell differentiation, with or without an encapsulation device, are currently tested in phase I/II clinical trials. As the pathogenesis of T1DM is remarkably similar between humans and dogs, novel treatment methods could be implemented in canine medicine. This review briefly summarises the physiology of the canine endocrine pancreas and the pathophysiology of canine DM before exploring current and possible future treatment options for canine DM.
Full article
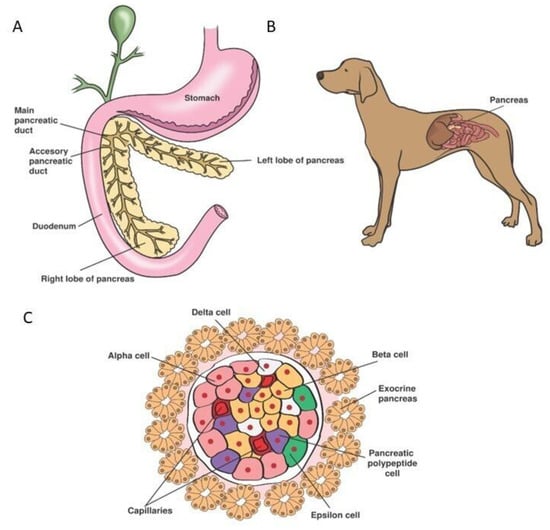
Figure 1
Open AccessProtocol
Generation of Trophoblast Organoids from Chorionic Villus Sampling
by
Bas van Rijn, Diane Van Opstal, Nicole van Koetsveld, Maarten Knapen, Joost Gribnau and Olivier Schäffers
Organoids 2024, 3(1), 54-66; https://doi.org/10.3390/organoids3010005 - 5 Mar 2024
Cited by 1
Abstract
►▼
Show Figures
Studying human placental development and function presents significant challenges due to the inherent difficulties in obtaining and maintaining placental tissue throughout the course of an ongoing pregnancy. Here, we provide a detailed protocol for generating trophoblast organoids from chorionic villi obtained during ongoing
[...] Read more.
Studying human placental development and function presents significant challenges due to the inherent difficulties in obtaining and maintaining placental tissue throughout the course of an ongoing pregnancy. Here, we provide a detailed protocol for generating trophoblast organoids from chorionic villi obtained during ongoing pregnancy. Our method results in efficient generation of trophoblast organoids from chorionic villus sampling, does not require preselection of chorionic villi, and controls contamination of decidual gland organoids. The resulting trophoblast organoids spontaneously form syncytiotrophoblasts that start secreting hCG hormone amongst other placenta-specific factors. Our approach facilitates the generation of trophoblast organoids from a variety of genetic backgrounds, including trisomies and gene mutations, and can be aligned with prenatal diagnostic routines. The protocol requires up to 14 days and can be carried out by users with expertise in cell culture.
Full article
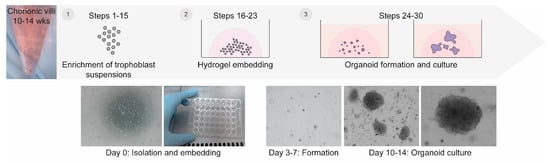
Figure 1
Open AccessArticle
Analysis of Osteosarcoma Cell Lines and Patient Tissue Using a 3D In Vivo Tumor Model—Possible Effects of Punicalagin
by
Anna Rebecca Dorn, Sara Neff, Sophia Hupp, Melissa Engelhardt, Eric Pion, Ulrich Lenze, Carolin Knebel, Anna Duprée, Simone Schewe, Markus Weber, Christian Wulbrand, Axel Hillmann, Florian Weber, Phillip Clarke, Philipp Kainz, Thiha Aung and Silke Haerteis
Organoids 2024, 3(1), 35-53; https://doi.org/10.3390/organoids3010004 - 4 Mar 2024
Cited by 1
Abstract
Osteosarcomas are the most common primary malignant bone tumors and mostly affect children, adolescents, and young adults. Despite current treatment options such as surgery and polychemotherapy, the survival of patients with metastatic disease remains poor. In recent studies, punicalagin has reduced the cell
[...] Read more.
Osteosarcomas are the most common primary malignant bone tumors and mostly affect children, adolescents, and young adults. Despite current treatment options such as surgery and polychemotherapy, the survival of patients with metastatic disease remains poor. In recent studies, punicalagin has reduced the cell viability, angiogenesis, and invasion in cell culture trials. The aim of this study was to examine the effects of punicalagin on osteosarcomas in a 3D in vivo tumor model. Human osteosarcoma biopsies and SaOs-2 and MG-63 cells, were grown in a 3D in vivo chorioallantoic membrane (CAM) model. After a cultivation period of up to 72 h, the tumors received daily treatment with punicalagin for 4 days. Weight measurements of the CAM tumors were performed, and laser speckle contrast imaging (LSCI) and a deep learning-based image analysis software (CAM Assay Application v.3.1.0) were used to measure angiogenesis. HE, Ki-67, and Caspase-3 staining was performed after explantation. The osteosarcoma cell lines SaOs-2 and MG-63 and osteosarcoma patient tissue displayed satisfactory growth patterns on the CAM. Treatment with punicalagin decreased tumor weight, proliferation, and tumor-induced angiogenesis, and the tumor tissue showed pro-apoptotic characteristics. These results provide a robust foundation for the implementation of further studies and show that punicalagin offers a promising supplementary treatment option for osteosarcoma patients. The 3D in vivo tumor model represents a beneficial model for the testing of anti-cancer therapies.
Full article
(This article belongs to the Special Issue Advanced Organoids: New Avenues for Understanding Human Anatomy, Physiology and Development)
►▼
Show Figures
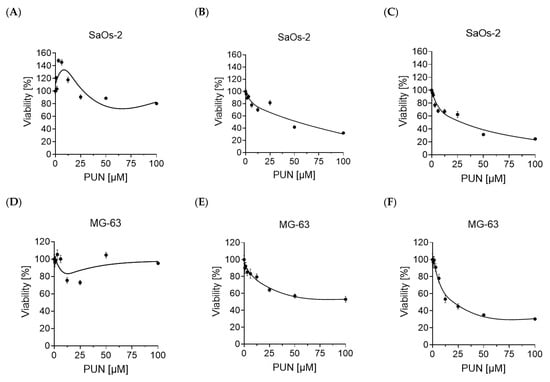
Figure 1
Open AccessEditorial
The Next Generation of Organoids Will Be More Complex and Even Closer to Resembling Real Organs: An Interview with Prof. Dr. Hans Clevers
by
Süleyman Ergün and Organoids Editorial Office
Organoids 2024, 3(1), 32-34; https://doi.org/10.3390/organoids3010003 - 20 Feb 2024
Cited by 1
Abstract
In this issue, we are pleased and honored to have an interview with Professor Hans Clevers, who is the Advisory Board Member of Organoids [...]
Full article
Open AccessArticle
Human Nasal Epithelium Organoids for Assessing Neutralizing Antibodies to a Protective SARS-CoV-2 Virus-like Particle Vaccine
by
Julio Carrera Montoya, Simon Collett, Daniel Fernandez Ruiz, Linda Earnest, Melissa A. Edeling, Ashley Huey Yiing Yap, Chinn Yi Wong, James P. Cooney, Kathryn C. Davidson, Jason Roberts, Steven Rockman, Bang M. Tran, Julie L. McAuley, Georgia Deliyannis, Samantha L. Grimley, Damian F. J. Purcell, Shafagh A. Waters, Dale I. Godfrey, Dhiraj Hans, Marc Pellegrini, Jason M. Mackenzie, Elizabeth Vincan, William R. Heath and Joseph Torresiadd
Show full author list
remove
Hide full author list
Organoids 2024, 3(1), 18-31; https://doi.org/10.3390/organoids3010002 - 1 Feb 2024
Abstract
►▼
Show Figures
Existing mRNA COVID-19 vaccines have shown efficacy in reducing severe cases and fatalities. However, their effectiveness against infection caused by emerging SARS-CoV-2 variants has waned considerably, necessitating the development of variant vaccines. Ideally, next-generation vaccines will be capable of eliciting broader and more
[...] Read more.
Existing mRNA COVID-19 vaccines have shown efficacy in reducing severe cases and fatalities. However, their effectiveness against infection caused by emerging SARS-CoV-2 variants has waned considerably, necessitating the development of variant vaccines. Ideally, next-generation vaccines will be capable of eliciting broader and more sustained immune responses to effectively counteract new variants. Additionally, in vitro assays that more closely represent virus neutralization in humans would greatly assist in the analysis of protective vaccine-induced antibody responses. Here, we present findings from a SARS-CoV-2 VLP vaccine encompassing three key structural proteins: Spike (S), Envelope (E), and Membrane (M). The VLP vaccine effectively produced neutralizing antibodies as determined by surrogate virus neutralization test, and induced virus-specific T-cell responses: predominantly CD4+, although CD8+ T cell responses were detected. T cell responses were more prominent with vaccine delivered with AddaVax compared to vaccine alone. The adjuvanted vaccine was completely protective against live virus challenge in mice. Furthermore, we utilized air–liquid-interface (ALI)-differentiated human nasal epithelium (HNE) as an in vitro system, which authentically models human SARS-CoV-2 infection and neutralization. We show that immune sera from VLP-vaccinated mice completely neutralized SARS-CoV-2 virus infection, demonstrating the potential of ALI-HNE to assess vaccine induced Nab.
Full article
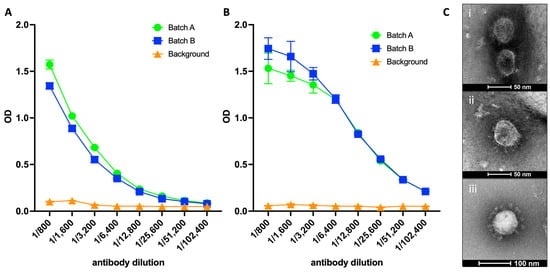
Figure 1
Open AccessFeature PaperArticle
Visualization of Vascular Perfusion of Human Pancreatic Cancer Tissue in the CAM Model and Its Impact on Future Personalized Drug Testing
by
Andreas Ettner-Sitter, Agata Montagner, Jonas Kuenzel, Kathrin Brackmann, Maximilian Schäfer, Robert Schober, Florian Weber, Thiha Aung, Christina Hackl and Silke Haerteis
Organoids 2024, 3(1), 1-17; https://doi.org/10.3390/organoids3010001 - 8 Jan 2024
Cited by 2
Abstract
Although significant improvements have been made in the treatment of pancreatic cancer, its prognosis remains poor with an overall 5-year survival rate of less than 10%. New experimental approaches are necessary to develop novel therapeutics. In this study, the investigation of pancreatic cancer
[...] Read more.
Although significant improvements have been made in the treatment of pancreatic cancer, its prognosis remains poor with an overall 5-year survival rate of less than 10%. New experimental approaches are necessary to develop novel therapeutics. In this study, the investigation of pancreatic cancer tissue growth in the chorioallantoic membrane (CAM) model and the subsequent use of indocyanine green (ICG) injections for the verification of intratumoral perfusion was conducted. ICG was injected into the CAM vasculature to visualize the perfusion of the tumor tissue. The presence of metastasis was investigated through PCR for the human-specific ALU element in the liver of the chicken embryo. Additionally, the usage of cryopreserved pancreatic tumors was established. Intratumoral perfusion of tumor tissue on the CAM was observed in recently obtained and cryopreserved tumors. ALU-PCR detected metastasis in the chick embryos’ livers. After cryopreservation, the tissue was still vital, and the xenografts generated from these tumors resembled the histological features of the primary tumor. This methodology represents the proof of principle for intravenous drug testing of pancreatic cancer in the CAM model. The cryopreserved tumors can be used for testing novel therapeutics and can be integrated into the molecular tumor board, facilitating personalized tumor treatment.
Full article
(This article belongs to the Special Issue Advanced Organoids: New Avenues for Understanding Human Anatomy, Physiology and Development)
►▼
Show Figures
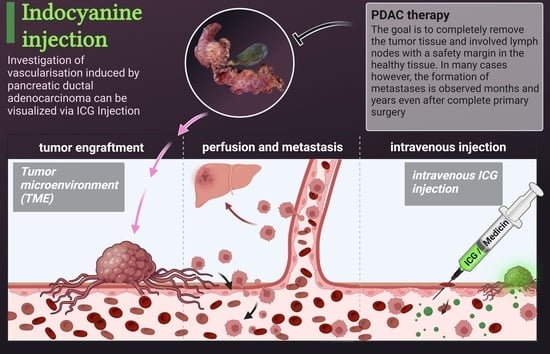
Graphical abstract
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Cells, Organoids, Cancers, Metabolites
Overview of Cancer Metabolism
Topic Editors: Arnaud Blomme, Cyril CorbetDeadline: 20 May 2025
Topic in
CIMB, IJMS, JCDD, Organoids, Biomedicines
Molecular and Cellular Mechanisms of Heart Disease
Topic Editors: Pasi Tavi, Ebru Arioglu-InanDeadline: 31 December 2026

Conferences
Special Issues
Special Issue in
Organoids
The Current Applications and Potential of Stem Cell-Derived Organoids
Guest Editors: James Adjaye, Nina GraffmannDeadline: 31 May 2025
Special Issue in
Organoids
Advances in Organoid Technology: Bridging the Gap between Research and Therapy
Guest Editors: Elizabeth Vincan, Ramanuj DasGupta, Somponnat Sampattavanich, Joao FerreiraDeadline: 30 June 2025
Special Issue in
Organoids
Organoid and Organ-on-a-Chip Research Advances in 2025
Guest Editors: Elvira Weber, Rhiannon David, Luc J. W. van der LaanDeadline: 31 July 2025








