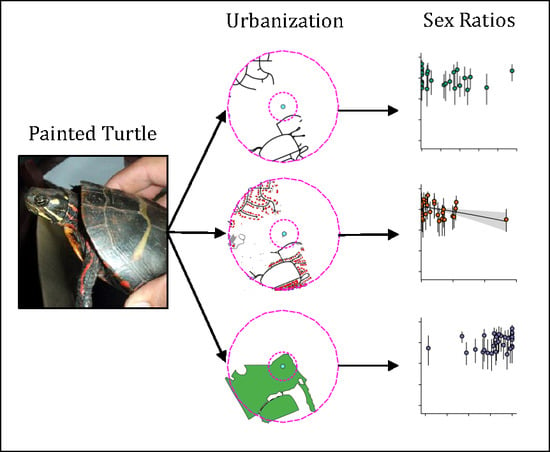
Assessing the Impacts of Urbanization on Sex Ratios of Painted Turtles (Chrysemys picta)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Organism
2.2. Study Location
2.3. Turtle Sampling
2.4. Landscape Characteristics
2.5. Data Analysis
3. Results
3.1. Turtle Sampling
3.2. Landscape Characteristics
3.3. Sex Ratios and Urbanization
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwarz, K.; Herrmann, D.L.; McHale, M.R. Abiotic drivers of ecologicla structure and function in urban ecosystems. In Urban Wildlife Conservation: Theory and Practice; McCleery, R.A., Moorman, C.E., Peterson, M.N., Eds.; Springer: New York, NY, USA, 2014; pp. 55–74. [Google Scholar]
- Oke, T.R. The energetic basis of the urban heat island. Q. J. R. Meteorol. Soc. 1982, 108, 1–24. [Google Scholar] [CrossRef]
- Arnfield, A.J. Two decades of urban climate research: A review of turbulence, exchanges of energy and water, and the urban heat island. Int. J. Climatol. 2003, 23, 1–26. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Mooney, H.A.; Lubchenco, J.; Melillo, J.M. Human domination of earth’s ecosystems. Science 1997, 277, 494–499. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization, biodiversity, and conservation. BioScience 2002, 52, 883–890. [Google Scholar] [CrossRef]
- Ditchkoff, S.S.; Saalfeld, S.T.; Gibson, C.J. Animal behavior in urban ecosystems: Modifications due to human-induced stress. Urban Ecosyst. 2006, 9, 5–12. [Google Scholar] [CrossRef]
- Prange, S.; Gehrt, S.D. Changes in mesopredator-community structure in response to urbanization. Can. J. Zool. 2004, 82, 1804–1817. [Google Scholar] [CrossRef]
- Czech, B.; Krausman, P.R.; Devers, P.K. Economic associations among causes of species endangerment in the United States. BioScience 2000, 50, 593–601. [Google Scholar] [CrossRef]
- Congdon, J.D.; Dunham, A.E.; Van Loben Sels, R.C. Delayed sexual maturity and demographics of Blanding’s turtles (Emydoidea blandingii): Implications for conservation and management of long-lived organisms. Conserv. Biol. 1993, 7, 826–833. [Google Scholar] [CrossRef]
- Mitchell, J.C.; Klemens, M.W. Primary and secondary effects of habitat alteration. In Turtle Conservation; Klemens, M.W., Ed.; Smithsonian Institution Press: Washington, DC, USA, 2000; pp. 5–32. ISBN 978-1-56098-372-9. [Google Scholar]
- Gibbs, J.P.; Shriver, W.G. Estimating the effects of road mortality on turtle populations. Conserv. Biol. 2002, 16, 1647–1652. [Google Scholar] [CrossRef]
- Congdon, J.D.; Dunham, A.E. Sels Demographics of common snapping turtles (Chelydra serpentina): implications for conservation and management of long-lived organisms. Am. Zool. 1994, 34, 397–408. [Google Scholar] [CrossRef]
- Minton, S.A. The fate of amphibians and reptiles in a suburban area. J. Herpetol. 1968, 2, 113–116. [Google Scholar] [CrossRef]
- Haxton, T. Road mortality of snapping turtles, Chelydra serpentina, in central Ontario during their nesting period. Can. Field-Nat. 2000, 114, 106–110. [Google Scholar]
- Aresco, M.J. Mitigation measures to reduce highway mortality of turtles and other herpetofauna at a North Florida lake. J. Wildl. Manag. 2005, 69, 549–560. [Google Scholar] [CrossRef]
- Wood, R.C.; Herlands, R. Turtles and tires: The impact of roadkills on northern diamondback terrapin, Malaclemys terrapin terrapin, populations on the Cape May peninsula, southern New Jersey, USA. In Proceedings: Conservation, Restoration, and Management of Tortoises and Turtles—An International Conference; New York Turtle & Tortoise Society: Mamaroneck, NY, USA, 1997; pp. 46–53. [Google Scholar]
- Feinberg, J.A.; Burke, R.L. Nesting ecology and predation of diamondback terrapins, Malaclemys terrapin, at Gateway National Recreation Area, New York. J. Herpetol. 2003, 37, 517–526. [Google Scholar] [CrossRef]
- Marchand, M.N.; Litvaitis, J.A. Effects of landscape composition, habitat features, and nest distribution on predation rates of simulated turtle nests. Biol. Conserv. 2004, 117, 243–251. [Google Scholar] [CrossRef]
- Browne, C.L.; Hecnar, S.J. Species loss and shifting population structure of freshwater turtles despite habitat protection. Biol. Conserv. 2007, 138, 421–429. [Google Scholar] [CrossRef]
- Ner, S.E.; Burke, R.L. Direct and indirect effects of urbanization on Diamond-backed Terrapins of the Hudson River Bight: Distribution and Predation in a human-modified estruary. In Urban Herpetology; Mitchell, J.C., Jung Brown, R.E., Bartholomew, B., Eds.; Herpetological Conservation; Society for the Study of Amphibians and Reptiles: Salt Lake City, UT, USA, 2008; Volume 3, pp. 121–143. [Google Scholar]
- Chen, M.; Zhang, H.; Liu, W.; Zhang, W. The global pattern of urbanization and economic growth: evidence from the last three decades. PLoS ONE 2014, 9, e103799. [Google Scholar] [CrossRef]
- Lovich, J.E.; Ennen, J.R.; Agha, M.; Gibbons, J.W. Where have all the turtles gone, and why does it matter? BioScience 2018, 68, 771–781. [Google Scholar] [CrossRef]
- Rhodin, A.G.J.; Stanford, C.B.; van Dijk, P.P.; Eisemberg, C.; Luiselli, L.; Mittermeier, R.A.; Hudson, R.; Horne, B.D.; Goode, E.V.; Kuchling, G.; et al. Global conservation status of turtles and tortoises (Order Testudines). Chelonian Conserv. Biol. 2018, 17, 135–161. [Google Scholar] [CrossRef]
- Marchand, M.N.; Litvaitis, J.A. Effects of habitat features and landscape composition on the population structure of a common aquatic turtle in a region undergoing rapid development. Conserv. Biol. 2004, 18, 758–767. [Google Scholar] [CrossRef]
- Steen, D.A.; Gibbs, J.P. Effects of roads on the structure of freshwater turtle populations. Conserv. Biol. 2004, 18, 1143–1148. [Google Scholar] [CrossRef]
- Aresco, M.J. The effect of sex-specific terrestrial movements and roads on the sex ratio of freshwater turtles. Biol. Conserv. 2005, 123, 37–44. [Google Scholar] [CrossRef]
- Gibbs, J.P.; Steen, D.A. Trends in sex ratios of turtles in the united states: implications of road mortality. Conserv. Biol. 2005, 19, 552–556. [Google Scholar]
- Steen, D.A.; Aresco, M.J.; Beilke, S.G.; Compton, B.W.; Condon, E.P.; Dodd, C.K.; Forrester, H.; Gibbons, J.W.; Greene, J.L.; Johnson, G.; et al. Relative vulnerability of female turtles to road mortality. Anim. Conserv. 2006, 9, 269–273. [Google Scholar] [CrossRef]
- Klemens, M.W. (Ed.) Turtle Conservation; Smithsonian Institution Press: Washington, DC, USA, 2000; ISBN 978-1-56098-372-9. [Google Scholar]
- Patrick, D.A.; Gibbs, J.P. Population structure and movements of freshwater turtles across a road-density gradient. Landsc. Ecol. 2010, 25, 791–801. [Google Scholar] [CrossRef]
- DeCatanzaro, R.; Chow-Fraser, P. Relationship of road density and marsh condition to turtle assemblage characteristics in the Laurentian Great Lakes. J. Great Lakes Res. 2010, 36, 357–365. [Google Scholar] [CrossRef]
- Laporte, M.; Silva Beaudry, C.-O.; Angers, B. Effects of road proximity on genetic diversity and reproductive success of the painted turtle (Chrysemys picta). Conserv. Genet. 2013, 14, 21–30. [Google Scholar] [CrossRef]
- Dorland, A.; Rytwinski, T.; Fahrig, L. Do Roads Reduce Painted Turtle (Chrysemys picta) Populations? PLoS ONE 2014, 9, e98414. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.N.; Peery, M.Z. Land use patterns skew sex ratios, decrease genetic diversity and trump the effects of recent climate change in an endangered turtle. Divers. Distrib. 2014, 20, 1425–1437. [Google Scholar] [CrossRef]
- Hamer, A.J.; Harrison, L.J.; Stokeld, D. Road density and wetland context alter population structure of a freshwater turtle. Austral Ecol. 2016, 41, 53–64. [Google Scholar] [CrossRef]
- Bowne, D.R.; Cosentino, B.J.; Anderson, L.J.; Bloch, C.P.; Cooke, S.; Crumrine, P.W.; Dallas, J.; Doran, A.; Dosch, J.J.; Druckenbrod, D.L.; et al. Effects of urbanization on the population structure of freshwater turtles across the United States. Conserv. Biol. 2018, 32, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.; Vogt, R. Temperature-dependent sex determination in turtles. Science 1979, 206, 1186–1188. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Steen, D.A. Reexamining effects of urbanization on population structure of freshwater turtles: Response to Browne et al. Conserv. Biol. 2018. In press. [Google Scholar]
- Gibbons, J.W.; Lovich, J.E. Where has turtle ecology been, and where is it going? Herpetologica 2019, 75, 4–20. [Google Scholar] [CrossRef]
- Chicago Region Biodiversity Council. Biodiversity Recovery Plan; Chicago Region Biodiversity Council: Chicago, IL, USA, 1999. [Google Scholar]
- Belovsky, G.E.; Botkin, D.B.; Crowl, T.A.; Cummins, K.W.; Franklin, J.F.; Hunter, M.L.; Joern, A.; Lindermayer, D.B.; MacMahon, J.A.; Margules, C.R.; et al. Ten suggestions to strengthen the science of ecology. BioScience 2004, 54, 345–351. [Google Scholar] [CrossRef]
- Ernst, C.H.; Lovich, J.E. Turtles of the United States and Canada, 2nd ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2009. [Google Scholar]
- US Census Bureau Data. Available online: https://www.census.gov/data.html (accessed on 22 March 2019).
- Illinois State Climatologist Waukegan Climate Normals. Available online: https://stateclimatologist.web.illinois.edu/data/climate-data/waukegan/ (accessed on 22 March 2019).
- Bowles, M.L.; Mcbride, J. Pre-European Settlement Vegetation of Lake County, Illinois. 2005, pp. 1–31. Available online: http://plantconservation.us/BowlesMcBrideLake.pdf (accessed on 25 April 2019).
- Homer, C.G.; Dewitz, J.A.; Yang, L.; Jin, S.; Danielson, P.; Xian, G.; Coulston, J.; Herold, N.D.; Wickham, J.D.; Megown, K. Completion of the 2011 National Land Cover Database for the conterminous United States-Representing a decade of land cover change information. Photogramm. Eng. Remote Sens. 2015, 81, 345–354. [Google Scholar]
- Ream, C.; Ream, R. The influence of sampling methods on the estimation of population structure in painted turtles. Am. Midl. Nat. 1966, 75, 325–338. [Google Scholar] [CrossRef]
- Gibbons, J.W. Sex ratios in turtles. Res. Popul. Ecol. 1970, 12, 252–254. [Google Scholar] [CrossRef]
- Frazer, N.B.; Gibbons, J.W.; Owens, T.J. Turtle trapping: preliminary tests of conventional wisdom. Copeia 1990, 1990, 1150–1152. [Google Scholar] [CrossRef]
- Gamble, T. The relative efficiency of basking and hoop traps for painted turtles (Chrysemys picta). Herpetol. Rev. 2006, 37, 308–312. [Google Scholar]
- Cagle, F.R. A system of marking turtles for future identification. Copeia 1939, 1939, 170–173. [Google Scholar] [CrossRef]
- Gibbons, J.W. Reproductive potential, activity, and cycles in the painted turtle, Chrysemys picta. Ecology 1968, 49, 399–409. [Google Scholar] [CrossRef]
- Moll, E.O. Latitudinal and intersubspecific variation in reproduction of the painted turtle, Chrysemys picta. Herpetologica 1973, 29, 307–318. [Google Scholar]
- Steen, D.A.; Gibbs, J.P.; Buhlmann, K.A.; Carr, J.L.; Compton, B.W.; Congdon, J.D.; Doody, J.S.; Godwin, J.C.; Holcomb, K.L.; Jackson, D.R.; et al. Terrestrial habitat requirements of nesting freshwater turtles. Biol. Conserv. 2012, 150, 121–128. [Google Scholar] [CrossRef] [Green Version]
- QGIS Development Team. QGIS Geographic Information System; Open Source Geospatial Foundation Project: Beaverton, OR, USA, 2018. [Google Scholar]
- Environmental Systems Research Institute. ArcGIS Desktop; Environmental Systems Research Institute: Redlands, CA, USA, 2018. [Google Scholar]
- Chicago Metropolitan Agency for Planning Data Hub High-Resolution Land Cover, NE Illinois and NW Indiana. 2010. Available online: https://datahub.cmap.illinois.gov/dataset/high-resolution-land-cover-ne-illinois-and-nw-indiana-2010 (accessed on 22 March 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- McDonald, J.H. Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014. [Google Scholar]
- Berges, J.A. Ratios, regression statistics, and “spurious” correlations. Limnol. Oceanogr. 1997, 42, 1006–1007. [Google Scholar] [CrossRef]
- Urbanek, R.E.; Glowacki, G.A.; Nielsen, C.K. Effect of raccoon (Procyon lotor) reduction on blanding’s turtle (Emydoidea blandingii) nest success. J. N. Am. Herpetol. 2016, 1, 39–44. [Google Scholar]
- Prange, S.; Gehrt, S.D.; Wiggers, E.P. Demographic Factors Contributing to High Raccoon Densities in Urban Landscapes. J. Wildl. Manag. 2003, 67, 324–333. [Google Scholar] [CrossRef]
- Francis, E.A.; Moldowan, P.D.; Greischar, M.A.; Rollinson, N. Anthropogenic nest sites provide warmer incubation environments than natural nest sites in a population of oviparous reptiles near their northern range limit. Oecologia 2019. [Google Scholar] [CrossRef]
- Garriga, N.; Santos, X.; Montori, A.; Richter-Boix, A.; Franch, M.; Llorente, G.A. Are protected areas truly protected? The impact of road traffic on vertebrate fauna. Biodivers. Conserv. 2012, 21, 2761–2774. [Google Scholar] [CrossRef]
- Mali, I.; Brown, D.J.; Ferrato, J.R.; Forstner, M.R.J. Sampling freshwater turtle populations using hoop nets: Testing potential biases. Wildl. Soc. Bull. 2014, 38, 580–585. [Google Scholar] [CrossRef]
- Moldowan, P.D.; Brooks, R.J.; Litzgus, J.D. Sex-biased seasonal capture rates in Painted Turtle (Chrysemys picta). Can. Field-Nat. 2018, 132, 20–24. [Google Scholar] [CrossRef]
- Thomas, R.B.; Vogrin, N.; Altig, R. Sexual and Seasonal Differences in Behavior of Trachemys scripta (Testudines: Emydidae). J. Herpetol. 1999, 33, 511–515. [Google Scholar] [CrossRef]
- Tesche, M.R.; Hodges, K.E. Unreliable population inferences from common trapping practices for freshwater turtles. Glob. Ecol. Conserv. 2015, 3, 802–813. [Google Scholar] [CrossRef] [Green Version]
- Hart, D.R. Dietary and Habitat Shift with Size of Red-Eared Turtles (Pseudemys scripta) in a Southern Louisiana Population. Herpetologica 1983, 39, 285–290. [Google Scholar]
- Rowe, J.W.; Dalgarn, S.F. Home range size and daily movements of midland painted turtles (Chrysemys picta marginata) in relation to body size, sex, and weather patterns. Herpetol. Conserv. Biol. 2010, 5, 461–473. [Google Scholar]
- Jaeger, C.P.; Cobb, V.A. Comparative spatial ecologies of female painted turtles (Chrysemys picta) and red-eared sliders (Trachemys scripta) at Teelfoot Lake, Tennessee. Chelonian Conserv. Biol. 2012, 11, 59–67. [Google Scholar]





| RD (254 m) | RD (1000 m) | IS (254 m) | IS (1000 m) | PA (254 m) | PA (1000 m) | |
|---|---|---|---|---|---|---|
| RD (254 m) | 0.52 | 0.44 | 0.05 | −0.36 | −0.37 | |
| RD (1000 m) | 0.52 | 0.32 | 0.37 | −0.27 | −0.50 | |
| IS (254 m) | 0.44 | 0.32 | 0.57 | −0.64 | −0.40 | |
| IS (1000 m) | 0.05 | 0.37 | 0.57 | −0.33 | −0.45 | |
| PA (254 m) | −0.36 | −0.27 | −0.64 | −0.33 | 0.56 | |
| PA (1000 m) | −0.37 | −0.50 | −0.40 | −0.45 | 0.56 |
| Plot ID | Scale (m) | # Male | # Female | # Adult | PM | RD (254 m) | RD (1000 m) | IS (254 m) | IS (1000 m) | PA (254 m) | PA (1000 m) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CUB-F-106 | 254 | 58 | 24 | 82 | 0.71 | 0.00 | NA | 0.00 | NA | 100.00 | NA |
| CUB-G-201 | 254 | 17 | 11 | 28 | 0.61 | 2.41 | NA | 2.79 | NA | 94.20 | NA |
| LAK-P-202 | 254 | 18 | 8 | 26 | 0.69 | 0.00 | NA | 0.69 | NA | 100.00 | NA |
| LAK-U-207 | 254 | 15 | 7 | 22 | 0.68 | 1.25 | NA | 2.59 | NA | 3.34 | NA |
| CUB-GF-61 | 1000 | 75 | 35 | 110 | 0.68 | NA | 4.91 | NA | 6.19 | NA | 63.38 |
| GRT-IF-37 | 1000 | 23 | 9 | 32 | 0.72 | NA | 4.75 | NA | 10.50 | NA | 45.39 |
| LAK-P-726 | 1000 | 44 | 11 | 55 | 0.80 | NA | 2.36 | NA | 5.15 | NA | 57.41 |
| LAK-UW-57 | 1000 | 31 | 9 | 40 | 0.78 | NA | 3.66 | NA | 3.00 | NA | 86.89 |
| ROL-CD-123 | 1000 | 47 | 13 | 60 | 0.78 | NA | 2.28 | NA | 5.46 | NA | 49.55 |
| ALM-B-201 | Both | 57 | 5 | 62 | 0.92 | 0.00 | 2.66 | 1.02 | 5.26 | 100.00 | 45.73 |
| CAH-A-201 | Both | 24 | 12 | 36 | 0.67 | 1.16 | 6.79 | 4.94 | 11.07 | 57.55 | 23.86 |
| CUB-B-101 | Both | 14 | 8 | 22 | 0.64 | 3.41 | 3.80 | 3.30 | 5.17 | 66.31 | 37.52 |
| CUB-H-107 | Both | 33 | 17 | 50 | 0.66 | 2.00 | 4.69 | 5.15 | 5.98 | 67.88 | 51.94 |
| DUC-C-201 | Both | 41 | 8 | 49 | 0.84 | 4.75 | 6.62 | 5.51 | 8.04 | 67.79 | 35.36 |
| ETH-E-104 | Both | 13 | 8 | 21 | 0.62 | 0.27 | 1.01 | 0.25 | 1.43 | 76.36 | 45.30 |
| FOX-A-101 | Both | 18 | 3 | 21 | 0.86 | 0.00 | 2.34 | 0.03 | 3.73 | 100.00 | 41.11 |
| FOX-E-102 | Both | 95 | 20 | 115 | 0.83 | 0.00 | 3.10 | 0.04 | 5.23 | 42.22 | 23.97 |
| GRS-D-102 | Both | 27 | 6 | 33 | 0.82 | 1.68 | 3.43 | 2.05 | 5.08 | 81.98 | 21.28 |
| GRT-IF-203 | Both | 23 | 9 | 32 | 0.72 | 0.50 | 4.75 | 1.37 | 10.50 | 90.00 | 44.90 |
| HER-E-102 | Both | 15 | 9 | 24 | 0.63 | 1.73 | 2.70 | 3.64 | 4.39 | 71.77 | 23.11 |
| LAK-A-103 | Both | 35 | 9 | 44 | 0.80 | 0.00 | 1.85 | 0.00 | 2.37 | 100.00 | 65.83 |
| LAK-F-105 | Both | 22 | 5 | 27 | 0.81 | 0.00 | 1.44 | 0.02 | 1.89 | 98.06 | 69.60 |
| LAK-F-106 | Both | 43 | 7 | 50 | 0.86 | 0.00 | 3.04 | 0.63 | 4.51 | 79.31 | 74.27 |
| LAK-L-112 | Both | 15 | 5 | 20 | 0.75 | 2.26 | 6.35 | 3.48 | 15.86 | 81.93 | 28.56 |
| LAK-T-121 | Both | 192 | 52 | 244 | 0.79 | 0.00 | 2.51 | 0.00 | 4.90 | 100.00 | 68.28 |
| LAK-U-203 | Both | 20 | 3 | 23 | 0.87 | 0.00 | 2.67 | 0.38 | 4.72 | 100.00 | 61.76 |
| MCD-DE-1_2 | Both | 24 | 6 | 30 | 0.80 | 0.28 | 5.29 | 0.90 | 12.94 | 95.37 | 38.16 |
| MID-B-103 | Both | 51 | 21 | 72 | 0.71 | 1.48 | 6.73 | 3.36 | 13.23 | 78.40 | 23.15 |
| MID-C-104 | Both | 20 | 4 | 24 | 0.83 | 0.32 | 6.72 | 0.37 | 10.09 | 87.24 | 32.93 |
| NIP-B-101 | Both | 21 | 10 | 31 | 0.68 | 0.00 | 3.01 | 0.00 | 9.20 | 97.96 | 38.46 |
| ROL-FG-67 | Both | 63 | 14 | 77 | 0.82 | 0.00 | 2.47 | 0.00 | 6.23 | 100.00 | 70.57 |
| SIN-B-103 | Both | 32 | 10 | 42 | 0.76 | 2.56 | 4.47 | 5.39 | 12.18 | 83.96 | 37.68 |
| SIN-I-102 | Both | 44 | 10 | 54 | 0.81 | 0.00 | 2.47 | 0.00 | 5.05 | 91.95 | 43.56 |
| WAD-B-102 | Both | 17 | 3 | 20 | 0.85 | 1.86 | 2.17 | 2.03 | 7.55 | 84.69 | 50.08 |
| WRI-B-101 | Both | 23 | 13 | 36 | 0.64 | 0.00 | 2.10 | 0.15 | 21.77 | 81.14 | 35.67 |
| WRI-B-102 | Both | 27 | 16 | 43 | 0.63 | 0.00 | 2.54 | 13.39 | 24.35 | 47.21 | 31.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanek, J.P.; Glowacki, G.A. Assessing the Impacts of Urbanization on Sex Ratios of Painted Turtles (Chrysemys picta). Diversity 2019, 11, 72. https://doi.org/10.3390/d11050072
Vanek JP, Glowacki GA. Assessing the Impacts of Urbanization on Sex Ratios of Painted Turtles (Chrysemys picta). Diversity. 2019; 11(5):72. https://doi.org/10.3390/d11050072
Chicago/Turabian StyleVanek, John P., and Gary A. Glowacki. 2019. "Assessing the Impacts of Urbanization on Sex Ratios of Painted Turtles (Chrysemys picta)" Diversity 11, no. 5: 72. https://doi.org/10.3390/d11050072
APA StyleVanek, J. P., & Glowacki, G. A. (2019). Assessing the Impacts of Urbanization on Sex Ratios of Painted Turtles (Chrysemys picta). Diversity, 11(5), 72. https://doi.org/10.3390/d11050072