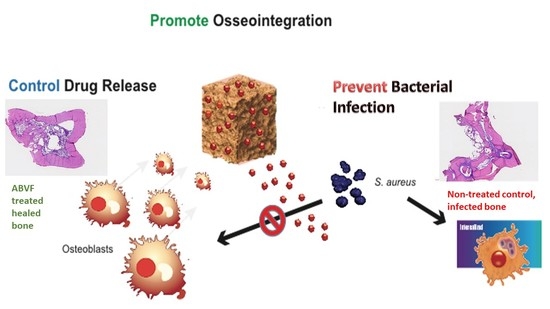
An Antibiotic-Releasing Bone Void Filling (ABVF) Putty for the Treatment of Osteomyelitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Vancomycin Free-Base (V-FB)
2.3. Fabrication and In Vitro Characterization
2.3.1. Preparation of ABVF
2.3.2. Scanning Electron Microscopy (SEM) of the ABVF Putty
2.3.3. Micro-Computed Tomography (μ-CT) of ABVF Putty
2.3.4. Mechanical Characterization
2.3.5. In Vitro Drug Release Kinetics
2.3.6. In Vitro Antibacterial Activity
2.3.7. In Vitro Cytocompatibility of ABVF Putty
2.4. In Vivo Assessment
2.4.1. Rat Osteomyelitis Model
2.4.2. Serum Creatinine
2.4.3. X-ray and μ-CT
2.4.4. Bone Volume
2.4.5. Histology
2.4.6. Bacterial Colony Count
2.4.7. Polymerase Chain Reaction (PCR)
2.5. Statistical Analysis
3. Results
3.1. Scanning Electron Microscopy (SEM) of the ABVF Putty
3.2. The μ-CT of ABVF Putty
3.3. Putty-Like Mechanical Property
3.4. In Vitro Vancomycin Release Kinetics
3.5. In Vitro Antibacterial Activity
3.6. In Vitro Cytocompatibility of ABVF Putty
3.7. In Vivo Study Results
3.7.1. Serum Creatinine
3.7.2. Radiographs—X-ray and μ-CT
3.7.3. Bone Volume Measurement
3.7.4. Histology
3.7.5. Bacterial Load
3.7.6. PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurtz, S.M.; Lau, E.; Schmier, J.; Ong, K.L.; Zhao, K.; Parvizi, J. Infection Burden for Hip and Knee Arthroplasty in the United States. J. Arthroplast. 2008, 23, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic Joint Infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jämsen, E.; Varonen, M.; Huhtala, H.; Lehto, M.U.K.; Lumio, J.; Konttinen, Y.T.; Moilanen, T. Incidence of Prosthetic Joint Infections After Primary Knee Arthroplasty. J. Arthroplast. 2010, 25, 87–92. [Google Scholar] [CrossRef]
- Sloan, M.; Sheth, N. Changing Demographics in Primary and Revision Total Joint Arthroplasty, 2000–2014. In Proceedings of the American Academy of Orthopaedic Surgeons 2018 Annual Meeting, New Orleans, LA, USA, 6–10 March 2018. [Google Scholar]
- AJRR_2016_Annual_Report_final.pdf. Available online: http://www.ajrr.net/images/annual_reports/AJRR_2016_Annual_Report_final.pdf (accessed on 13 March 2018).
- Lamagni, T. Epidemiology and burden of prosthetic joint infections. J. Antimicrob. Chemother. 2014, 69, i5–i10. [Google Scholar] [CrossRef] [Green Version]
- CNN, S.S. More Men, Younger Americans Having Joint Replacement Surgery. Available online: https://www.cnn.com/2018/03/06/health/hip-knee-replacement-surgeries-earlier-study/index.html (accessed on 14 March 2018).
- Mortazavi, S.M.J.; Schwartzenberger, J.; Austin, M.S.; Purtill, J.J.; Parvizi, J. Revision Total Knee Arthroplasty Infection: Incidence and Predictors. Clin. Orthop. Relat. Res. 2010, 468, 2052–2059. [Google Scholar] [CrossRef] [Green Version]
- Conterno, L.O.; Da Silva Filho, C.R. Antibiotics for treating chronic osteomyelitis in adults. Cochrane Database Syst. Rev. 2013, 9. [Google Scholar] [CrossRef]
- Landersdorfer, C.B.; Bulitta, J.B.; Kinzig, M.; Holzgrabe, U.; Sörgel, F. Penetration of Antibacterials into Bone. Clin. Pharmacokinet. 2009, 48, 89–124. [Google Scholar] [CrossRef]
- Liu, X.-M.; Zhang, Y.; Chen, F.; Khutsishvili, I.; Fehringer, E.V.; Marky, L.A.; Bayles, K.W.; Wang, D. Prevention of Orthopedic Device-Associated Osteomyelitis Using Oxacillin-Containing Biomineral-Binding Liposomes. Pharm. Res. 2012, 29, 3169–3179. [Google Scholar] [CrossRef] [Green Version]
- Ter Boo, G.-J.A.; Grijpma, D.W.; Moriarty, T.F.; Richards, R.G.; Eglin, D. Antimicrobial delivery systems for local infection prophylaxis in orthopedic- and trauma surgery. Biomaterials 2015, 52, 113–125. [Google Scholar] [CrossRef]
- Haidar, R.; Boghossian, A.D.; Atiyeh, B. Duration of post-surgical antibiotics in chronic osteomyelitis: Empiric or evidence-based? Int. Infect. Dis. 2010, 14, e752–e758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biodegradable Mg-Cu Alloy Implants with Antibacterial Activity for the Treatment of Osteomyelitis: In Vitro and In Vivo Evaluations. - PubMed - NCBI. Available online: https://www.ncbi.nlm.nih.gov/pubmed/27573133 (accessed on 24 November 2018).
- Anagnostakos, K.; Fürst, O.; Kelm, J. Antibiotic-impregnated PMMA hip spacers: Current status. Acta Orthop. 2006, 77, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Oungeun, P.; Rojanathanes, R.; Pinsornsak, P.; Wanichwecharungruang, S. Sustaining Antibiotic Release from a Poly(methyl methacrylate) Bone-Spacer. ACS Omega 2019, 4, 14860–14867. [Google Scholar] [CrossRef] [PubMed]
- Sanicola, S.M.; Albert, S.F. The in vitro elution characteristics of vancomycin and tobramycin from calcium sulfate beads. J. Foot Ankle Surg. 2005, 44, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Van Vugt, T.A.G.; Arts, J.J.; Geurts, J.A.P. Antibiotic-Loaded Polymethylmethacrylate Beads and Spacers in Treatment of Orthopedic Infections and the Role of Biofilm Formation. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Y.; Liu, Y.; Yan, L.; Zaat, S.A.J.; Wismeijer, D.; Pathak, J.L.; Wu, G. A dual functional bone-defect-filling material with sequential antibacterial and osteoinductive properties for infected bone defect repair. J. Biomed. Mater. Res. Part A 2019, 107, 2360–2370. [Google Scholar] [CrossRef]
- Thomes, B.; Murray, P.; Bouchier-Hayes, D. Development of resistant strains of Staphylococcus epidermidis on gentamicin-loaded bone cement in vivo. J. Bone Jt. Surg. Br. 2002, 84, 758–760. [Google Scholar] [CrossRef] [Green Version]
- Kendall, R.W.; Duncan, C.P.; Smith, J.A.; Ngui-Yen, J.H. Persistence of bacteria on antibiotic loaded acrylic depots: A reason for caution. Clin. Orthop. Relat. Res. 1996, 273–280. [Google Scholar] [CrossRef]
- Minelli, E.B.; Della Bora, T.; Benini, A. Different microbial biofilm formation on polymethylmethacrylate (PMMA) bone cement loaded with gentamicin and vancomycin. Anaerobe 2011, 17, 380–383. [Google Scholar] [CrossRef]
- Miller, A.J.; Stimac, J.D.; Smith, L.S.; Feher, A.W.; Yakkanti, M.R.; Malkani, A.L. Results of Cemented vs Cementless Primary Total Knee Arthroplasty Using the Same Implant Design. J. Arthroplast. 2018, 33, 1089–1093. [Google Scholar] [CrossRef]
- Schachter, D. Bone Void Filler; US20060067973A1; Ethicon Inc.: Somerville, NI, USA, 2006. [Google Scholar]
- Pryor, L.S.; Gage, E.; Langevin, C.-J.; Herrera, F.; Breithaupt, A.D.; Gordon, C.R.; Afifi, A.M.; Zins, J.E.; Meltzer, H.; Gosman, A.; et al. Review of Bone Substitutes. Craniomaxillofac. Trauma Reconstr. 2009, 2, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VICKERS, S.M.; Scifert, J.L. Flowable Paste and Putty Bone Void Filler; US8653029B2; Warsaw Orthopedic Inc.: Warsaw, IN, USA, 2014. [Google Scholar]
- Puga, A.M.; Rey-Rico, A.; Magariños, B.; Alvarez-Lorenzo, C.; Concheiro, A. Hot melt poly-ε-caprolactone/poloxamine implantable matrices for sustained delivery of ciprofloxacin. Acta Biomater. 2012, 8, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Fraimow, H.S. Systemic Antimicrobial Therapy in Osteomyelitis. Semin. Plast. Surg. 2009, 23, 90–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chohfi, M.; Langlais, F.; Fourastier, J.; Minet, J.; Thomazeau, H.; Cormier, M. Pharmacokinetics, uses, and limitations of vancomycin-loaded bone cement. Int. Orthop. 1998, 22, 171–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, C.C.; Sousa, S.R.; Monteiro, F.J. Heparinized nanohydroxyapatite/collagen granules for controlled release of vancomycin. J. Biomed. Mater. Res. 2015, 103, 3128–3138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graziani, A.L.; Lawson, L.A.; Gibson, G.A.; Steinberg, M.A.; MacGregor, R.R. Vancomycin concentrations in infected and noninfected human bone. Antimicrob. Agents Chemother. 1988, 32, 1320–1322. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Bernice, F. Vancomycin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Li, B.; Brown, K.V.; Wenke, J.C.; Guelcher, S.A. Sustained release of vancomycin from polyurethane scaffolds inhibits infection of bone wounds in a rat femoral segmental defect model. J. Control. Release 2010, 145, 221–230. [Google Scholar] [CrossRef]
- Curley, J.; Hasan, M.R.; Larson, J.; Brooks, B.D.; Liu, Q.; Jain, T.; Joy, A.; Brooks, A.E. An Osteoconductive Antibiotic Bone Eluting Putty with a Custom Polymer Matrix. Polymers 2016, 8, 247. [Google Scholar] [CrossRef] [Green Version]
- Chomczynski, P.; Mackey, K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. BioTechniques 1995, 19, 942–945. [Google Scholar]
- TRIZOLRNAIsolation_092107_21453_284_10813_v1.pdf. Available online: https://medicine.yale.edu/keck/ycga/microarrays/protocols/TRIZOLRNAIsolation_092107_21453_284_10813_v1.pdf (accessed on 14 September 2018).
- Stacer, R.G.; Husband, D.M.; Stacer, H.L. Viscoelastic Response and Adhesion Properties of Highly Filled Elastomers. Rubber Chem. Technol. 1987, 60, 227–244. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Ganji, F. Vancomycin-loaded HPMC microparticles embedded within injectable thermosensitive chitosan hydrogels. Prog. Biomater. 2017, 6, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinho, D.S.; Huf, G.; Ferreira, B.L.; Castro, H.; Rodrigues, C.R.; De Sousa, V.P.; Cabral, L.M. The study of vancomycin use and its adverse reactions associated to patients of a brazilian university hospital. BMC Res. Notes 2011, 4, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahl, P.; Guidi, M.; Benninger, E.; Rönn, K.; Gautier, E.; Buclin, T.; Magnin, J.-L.; Livio, F. The levels of vancomycin in the blood and the wound after the local treatment of bone and soft-tissue infection with antibiotic-loaded calcium sulphate as carrier material. Bone Jt. J. 2017, 99-B, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Bolton, W.K.; Benton, F.R.; Maclay, J.G.; Sturgill, B.C. Spontaneous Glomerular Sclerosis in Aging Sprague-Dawley Rats. Am. J. Pathol. 1976, 85, 277. [Google Scholar]
- sprague-dawley-rat.pdf. Available online: https://www.taconic.com/pdfs/sprague-dawley-rat.pdf (accessed on 14 September 2018).
- Mader, J.T.; Calhoun, J. Bone, Joint, and Necrotizing Soft Tissue Infections. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; ISBN 978-0-9631172-1-2. [Google Scholar]
- Carli, A.V.; Sethuraman, A.S.; Bhimani, S.J.; Ross, F.P.; Bostrom, M.P.G. Selected Heat-Sensitive Antibiotics Are Not Inactivated during Polymethylmethacrylate Curing and Can Be Used in Cement Spacers for Periprosthetic Joint Infection. J. Arthroplast. 2018, 33, 1930–1935. [Google Scholar] [CrossRef]
- Hafeman, A.E.; Zienkiewicz, K.J.; Carney, E.; Litzner, B.; Stratton, C.; Wenke, J.C.; Guelcher, S.A. Local Delivery of Tobramycin from Injectable Biodegradable Polyurethane Scaffolds. J. Biomater. Sci. Polym. Ed. 2010, 21, 95–112. [Google Scholar] [CrossRef] [Green Version]
- Hasan, R.; Brooks, A. Novel Local Bone Void Filling Antibiotic Carriers for the Treatment of Bone Infection. Ortho. Res. Online J. 2017, 1. [Google Scholar] [CrossRef] [Green Version]
- Aiken, S.S.; Cooper, J.J.; Florance, H.; Robinson, M.T.; Michell, S. Local Release of Antibiotics for Surgical Site Infection Management Using High-Purity Calcium Sulfate: An In Vitro Elution Study. Surg. Infect. (Larchmt) 2015, 16, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Scharer, B.M.; Sanicola, S.M. The In Vitro Elution Characteristics of Vancomycin from Calcium Phosphate–Calcium Sulfate Beads. J. Foot Ankle Surg. 2009, 48, 540–542. [Google Scholar] [CrossRef]
- Dorati, R.; De Trizio, A.; Genta, I.; Merelli, A.; Modena, T.; Conti, B. Gentamicin-Loaded Thermosetting Hydrogel and Moldable Composite Scaffold: Formulation Study and Biologic Evaluation. J. Pharm. Sci. 2017, 106, 1596–1607. [Google Scholar] [CrossRef]
- Dorati, R.; De Trizio, A.; Genta, I.; Merelli, A.; Modena, T.; Conti, B. Formulation and in vitro characterization of a composite biodegradable scaffold as antibiotic delivery system and regenerative device for bone. J. Drug Deliv. Sci. Technol. 2016, 35, 124–133. [Google Scholar] [CrossRef]
- Padrão, T.; Coelho, C.C.; Costa, P.; Alegrete, N.; Monteiro, F.J.; Sousa, S.R. Combining local antibiotic delivery with heparinized nanohydroxyapatite/collagen bone substitute: A novel strategy for osteomyelitis treatment. Mater. Sci. Eng. C 1920, 119, 111329. [Google Scholar] [CrossRef]
- Le Ray, A.-M.; Gautier, H.; Laty, M.-K.; Daculsi, G.; Merle, C.; Jacqueline, C.; Hamel, A.; Caillon, J. In Vitro and In Vivo Bactericidal Activities of Vancomycin Dispersed in Porous Biodegradable Poly(ɛ-Caprolactone) Microparticles. Antimicrob. Agents Chemother. 2005, 49, 3025–3027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inzana, J.A.; Trombetta, R.P.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printed bioceramics for dual antibiotic delivery to treat implant-associated bone infection. Eur. Cells Mater. 2015, 30, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Guelcher, S.A.; Brown, K.V.; Li, B.; Guda, T.; Lee, B.-H.; Wenke, J.C. Dual-purpose bone grafts improve healing and reduce infection. J. Orthop. Trauma 2011, 25, 477–482. [Google Scholar] [CrossRef]
- Chen, X.; Kidder, L.S.; Lew, W.D. Osteogenic protein-1 induced bone formation in an infected segmental defect in the rat femur. J. Orthop. Res. 2002, 20, 142–150. [Google Scholar] [CrossRef]
- Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials 2016, 81, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Subbiahdoss, G.; Kuijer, R.; Grijpma, D.W.; Van der Mei, H.C.; Busscher, H.J. Microbial biofilm growth vs. tissue integration: “The race for the surface” experimentally studied. Acta Biomater. 2009, 5, 1399–1404. [Google Scholar] [CrossRef]
- Benito, N.; Esteban, J.; Horcajada, J.P.; Ribera, A.; Soriano, A.; Sousa, R. Epidemiology of Prosthetic Joint Infection. In Prosthetic Joint Infections; Peel, T., Ed.; Springer International Publishing: Cham, Switerland, 2018; pp. 5–53. ISBN 9783319652504. [Google Scholar]
- Prieto, E.M.; Talley, A.D.; Gould, N.R.; Zienkiewicz, K.J.; Drapeau, S.J.; Kalpakci, K.N.; Guelcher, S.A. Effects of Particle Size and Porosity on In Vivo Remodeling of Settable Allograft Bone/Polymer Composites. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1641–1651. [Google Scholar] [CrossRef] [Green Version]
- Shapoff, C.A.; Bowers, G.M.; Levy, B.; Mellonig, J.T.; Yukna, R.A. The effect of particle size on the osteogenic activity of composite grafts of allogeneic freeze-dried bone and autogenous marrow. J. Periodontol. 1980, 51, 625–630. [Google Scholar] [CrossRef]
- Coathup, M.J.; Cai, Q.; Campion, C.; Buckland, T.; Blunn, G.W. The effect of particle size on the osteointegration of injectable silicate-substituted calcium phosphate bone substitute materials. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101B, 902–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, F.; Du, C.; Yu, G.; Li, R. The Static and Dynamic Mechanical Properties of Magnetorheological Silly Putty. Available online: https://www.hindawi.com/journals/amse/2016/7079698/ (accessed on 19 September 2018).
- Solorio, L.; Babin, B.M.; Patel, R.B.; Mach, J.; Azar, N.; Exner, A.A. Noninvasive Characterization of In situ Forming Implants Using Diagnostic Ultrasound. J. Control. Release 2010, 143, 183–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Ingredients | PRO OSTEON | V-HCl | V-FB | PLGA | PCL | PEG | CaCl2 |
|---|---|---|---|---|---|---|---|
| % amount | 51.72 | 14.78 | 8.20 | 12.63 | 6.28 | 3.13 | 3.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, R.; Wohlers, A.; Shreffler, J.; Mulinti, P.; Ostlie, H.; Schaper, C.; Brooks, B.; Brooks, A. An Antibiotic-Releasing Bone Void Filling (ABVF) Putty for the Treatment of Osteomyelitis. Materials 2020, 13, 5080. https://doi.org/10.3390/ma13225080
Hasan R, Wohlers A, Shreffler J, Mulinti P, Ostlie H, Schaper C, Brooks B, Brooks A. An Antibiotic-Releasing Bone Void Filling (ABVF) Putty for the Treatment of Osteomyelitis. Materials. 2020; 13(22):5080. https://doi.org/10.3390/ma13225080
Chicago/Turabian StyleHasan, Raquib, Abbey Wohlers, Jacob Shreffler, Pranothi Mulinti, Hunter Ostlie, Codi Schaper, Benjamin Brooks, and Amanda Brooks. 2020. "An Antibiotic-Releasing Bone Void Filling (ABVF) Putty for the Treatment of Osteomyelitis" Materials 13, no. 22: 5080. https://doi.org/10.3390/ma13225080
APA StyleHasan, R., Wohlers, A., Shreffler, J., Mulinti, P., Ostlie, H., Schaper, C., Brooks, B., & Brooks, A. (2020). An Antibiotic-Releasing Bone Void Filling (ABVF) Putty for the Treatment of Osteomyelitis. Materials, 13(22), 5080. https://doi.org/10.3390/ma13225080