Cellulose-Acetate-Based Films Modified with Ag2O and ZnS as Nanocomposites for Highly Controlling Biological Behavior for Wound Healing Applications
Abstract
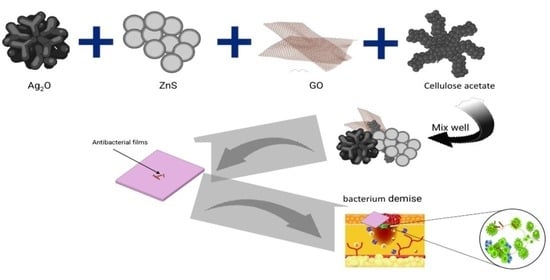
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.3. Characterization Methods
2.4. The Water Contact Angle
2.5. Thermal Gravimetric Analysis (TGA)
2.6. UV-Vis Spectrophotometer
2.7. Cytotoxicity Test
2.8. Antibacterial Measurement
3. Results and Discussions
3.1. XRD
3.2. FTIR
3.3. SEM/EDX Analysis
3.4. Contact Angle
3.5. TGA
3.6. Cytotoxicity
3.7. Uv-Vis
3.8. Antibacterial
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakai, R.; John, B.; Okamoto, M.; Seppälä, J.V.; Vaithilingam, J.; Hussein, H.; Goodridge, R. Fabrication of polylactide-based biodegradable thermoset scaffolds for tissue engineering applications. Macromol. Mater. Eng. 2013, 298, 45–52. [Google Scholar] [CrossRef]
- Atila, D.; Keskin, D.; Tezcaner, A. Crosslinked pullulan/cellulose acetate fibrous scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2016, 69, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sudhakara, P.; Singh, J.; Ilyas, R.; Asyraf, M.; Razman, M. Critical review of biodegradable and bioactive polymer composites for bone tissue engineering and drug delivery applications. Polymers 2021, 13, 2623. [Google Scholar] [CrossRef]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and synthetic polymers for bone scaffolds optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef] [Green Version]
- Janoušková, O. Synthetic polymer scaffolds for soft tissue engineering. Physiol. Res. 2018, 67 (Suppl. S2), S335–S348. [Google Scholar] [CrossRef]
- Dirauf, M.; Muljajew, I.; Weber, C.; Schubert, U.S. Recent advances in degradable synthetic polymers for biomedical applications—Beyond polyesters. Prog. Polym. Sci. 2022, 129, 101547. [Google Scholar] [CrossRef]
- Hogan, K.J.; Mikos, A.G. Biodegradable thermoresponsive polymers: Applications in drug delivery and tissue engineering. Polymer 2020, 211, 123063. [Google Scholar] [CrossRef]
- Yadav, N.; Hakkarainen, M. Degradable or not? Cellulose acetate as a model for complicated interplay between structure, environment and degradation. Chemosphere 2021, 265, 128731. [Google Scholar] [CrossRef]
- Tan, H.-L.; Kai, D.; Pasbakhsh, P.; Teow, S.-Y.; Lim, Y.-Y.; Pushpamalar, J. Electrospun cellulose acetate butyrate/polyethylene glycol (CAB/PEG) composite nanofibers: A potential scaffold for tissue engineering. Colloids Surf. B Biointerfaces 2020, 188, 110713. [Google Scholar] [CrossRef]
- Rad, R.M.; Alshemary, A.Z.; Evis, Z.; Keskin, D.; Tezcaner, A. Cellulose acetate-gelatin-coated boron-bioactive glass biocomposite scaffolds for bone tissue engineering. Biomed. Mater. 2020, 15, 065009. [Google Scholar]
- Kalaycıoğlu, Z.; Kahya, N.; Adımcılar, V.; Kaygusuz, H.; Torlak, E.; Akın-Evingür, G.; Erim, F.B. Antibacterial nano cerium oxide/chitosan/cellulose acetate composite films as potential wound dressing. Eur. Polym. J. 2020, 133, 109777. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Hussein, M.A.; Al-Hadeethi, Y.; Umar, A. Cellulose acetate-hydroxyapatite-bioglass-zirconia nanocomposite particles as potential biomaterial: Synthesis, characterization, and biological properties for bone application. Eng. Sci. 2021, 17, 70–82. [Google Scholar] [CrossRef]
- Tsiapla, A.-R.; Karagkiozaki, V.; Bakola, V.; Pappa, F.; Gkertsiou, P.; Pavlidou, E.; Logothetidis, S. Biomimetic and biodegradable cellulose acetate scaffolds loaded with dexamethasone for bone implants. Beilstein J. Nanotechnol. 2018, 9, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, A.; Ghazali, M.J.; Mahmoudi, E.; Azizkhani, S.; Sulaiman, N.H.; Mohammad, A.W.; Mustafah, N.M.; Ohnmar, H.; Naicker, A.S. Development and antibacterial application of nanocomposites: Effects of molar ratio on Ag2O–CuO nanocomposite synthesised via the microwave-assisted route. Ceram. Int. 2018, 44, 21591–21598. [Google Scholar] [CrossRef]
- Rajabi, A.; Ghazali, M.J.; Mahmoudi, E.; Baghdadi, A.H.; Mohammad, A.W.; Mustafah, N.M.; Ohnmar, H.; Naicker, A.S. Synthesis, Characterization, and Antibacterial Activity of Ag2O-Loaded Polyethylene Terephthalate Fabric via Ultrasonic Method. Nanomaterials 2019, 9, 450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, B.; Fang, W.H.; Zhao, S.; Yang, Z.; Hoang, B.X. Zinc sulfide nanoparticles improve skin regeneration. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102263. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, W.; Tian, P.; Meng, F.; Zhu, H.; Jiang, X.; Liu, X.; Chu, P.K. Stimulation of bone growth following zinc incorporation into biomaterials. Biomaterials 2014, 35, 6882–6897. [Google Scholar] [CrossRef]
- Guo, W.; Kan, J.-T.; Cheng, Z.-Y.; Chen, J.-F.; Shen, Y.-Q.; Xu, J.; Wu, D.; Zhu, Y.-Z. Hydrogen sulfide as an endogenous modulator in mitochondria and mitochondria dysfunction. Oxidative Med. Cell Longev. 2012, 2012, 878052. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.; Raizada, P.; Hasija, V.; Singh, P.; Bajpai, A.; Nguyen, V.-H.; Rangabhashiyam, S.; Kumar, P.; Nadda, A.K.; Kim, S.Y.; et al. ZnS-based quantum dots as photocatalysts for water purification. J. Water Process Eng. 2021, 43, 102217. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, H.; Chen, J.; Lv, Y.; Dong, C.; Sreeprasad, T.S. Graphene and graphene oxide: Advanced membranes for gas separation and water purification. Inorg. Chem. Front. 2015, 2, 417–424. [Google Scholar] [CrossRef]
- Feng, P.; Jia, J.; Peng, S.; Yang, W.; Bin, S.; Shuai, C. Graphene oxide-driven interfacial coupling in laser 3D printed PEEK/PVA scaffolds for bone regeneration. Virtual Phys. Prototyp. 2020, 15, 211–226. [Google Scholar] [CrossRef]
- Zhou, M.; Lozano, N.; Wychowaniec, J.K.; Hodgkinson, T.; Richardson, S.M.; Kostarelos, K.; Hoyland, J.A. Graphene oxide: A growth factor delivery carrier to enhance chondrogenic differentiation of human mesenchymal stem cells in 3D hydrogels. Acta Biomater. 2019, 96, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Zarezadeh, R.; Nouri, M.; Sadigh, A.R.; Pouremamali, F.; Asemi, Z.; Kafil, H.S.; Alemi, F.; Yousefi, B. Graphene oxide: A promising material for regenerative medicine and tissue engineering. Biomol. Concepts 2020, 11, 182–200. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.; Bakhtiari, S.S.E.; Karbasi, S. Incorporation of chitosan/graphene oxide nanocomposite in to the PMMA bone cement: Physical, mechanical and biological evaluation. Int. J. Biol. Macromol. 2020, 149, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Purohit, S.D.; Bhaskar, R.; Singh, H.; Yadav, I.; Gupta, M.K.; Mishra, N.C. Development of a nanocomposite scaffold of gelatin–alginate–graphene oxide for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 592–602. [Google Scholar] [CrossRef]
- Li, T.-T.; Sun, L.; Zhong, Y.; Peng, H.-K.; Ren, H.-T.; Zhang, Y.; Lin, J.-H.; Lou, C.-W. Silk fibroin/polycaprolactone-polyvinyl alcohol directional moisture transport composite film loaded with antibacterial drug-loading microspheres for wound dressing materials. Int. J. Biol. Macromol. 2022, 207, 580–591. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.-T.; Shiu, B.-C.; Lin, J.-H.; Lou, C.-W. Multifunctional sodium Alginate@ urushiol fiber with targeted Antibacterial, acid corrosion resistance and flame retardant properties for personal protection based on wet spinning. Appl. Surf. Sci. 2022, 584, 152573. [Google Scholar] [CrossRef]
- Sharma, A.; Mandal, T.; Goswami, S. Fabrication of cellulose acetate nanocomposite films with lignocelluosic nanofiber filler for superior effect on thermal, mechanical and optical properties. Nano-Struct. Nano-Objects 2021, 25, 100642. [Google Scholar] [CrossRef]
- Huda, E. Preparation and characterization of cellulose acetate from cotton. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Moscow, Russia, 27 May–6 June 2019; p. 012021. [Google Scholar]
- Vetrivel, S.; Saraswathi, M.S.S.A.; Rana, D.; Divya, K.; Nagendran, A. Cellulose acetate ultrafiltration membranes customized with copper oxide nanoparticles for efficient separation with antifouling behavior. J. Appl. Polym. Sci. 2021, 138, 49867. [Google Scholar] [CrossRef]
- Chakraborty, U.; Bhanjana, G.; Kaur, G.; Kaushik, A.; Chaudhary, G.R. Electro-active silver oxide nanocubes for label free direct sensing of bisphenol A to assure water quality. Mater. Today Chem. 2020, 16, 100267. [Google Scholar] [CrossRef]
- De, A.K.; Majumdar, S.; Pal, S.; Kumar, S.; Sinha, I. Zn doping induced band gap widening of Ag2O nanoparticles. J. Alloys Compd. 2020, 832, 154127. [Google Scholar] [CrossRef]
- Prasad, N.; Karthikeyan, B. A Raman spectral probe on polar w-ZnS nanostructures and surface optical phonon modes in nanowires. Nanoscale 2019, 11, 4948–4958. [Google Scholar] [CrossRef] [PubMed]
- Trung, D.; Tran, M.; Tu, N.; Thu, L.; Huyen, N.; Hung, N.; Viet, D.; Kien, N.; Huy, P. Synthesis, structural and optical properties of ZnS/ZnO heterostructure-alloy hexagonal micropyramids. Opt. Mater. 2022, 125, 112077. [Google Scholar] [CrossRef]
- Suhaimi, L.; Az-Zahra, A.; Pradipta, O.; Shidqi, D. The Fabrication of Cellulose Acetate Fiber based on Empty Fruit Bunches (EFB) using Electrospinning Technique. J. Kim. Terap. Indones. 2022, 24, 15–22. [Google Scholar]
- Liu, L.; Gong, D.; Bratasz, L.; Zhu, Z.; Wang, C. Degradation markers and plasticizer loss of cellulose acetate films during ageing. Polym. Degrad. Stab. 2019, 168, 108952. [Google Scholar] [CrossRef]
- Homem, N.C.; Amorim, M.T.P. Synthesis of cellulose acetate using as raw material textile wastes. Mater. Today Proc. 2020, 31, S315–S317. [Google Scholar] [CrossRef]
- Sofi, H.S.; Akram, T.; Shabir, N.; Vasita, R.; Jadhav, A.H.; Sheikh, F.A. Regenerated cellulose nanofibers from cellulose acetate: Incorporating hydroxyapatite (HAp) and silver (Ag) nanoparticles (NPs), as a scaffold for tissue engineering applications. Mater. Sci. Eng. C 2021, 118, 111547. [Google Scholar] [CrossRef]
- Athira, V.B.; Mohanty, S.; Nayak, S.K. Preparation and characterization of porous polyethersulfone (PES) membranes with improved biocompatibility by blending sulfonated polyethersulfone (SPES) and cellulose acetate (CA)—A comparative study. Mater. Today Commun. 2020, 25, 101544. [Google Scholar] [CrossRef]
- Asriza, R.; Humaira, D.; Ryaldi, G. Characterization of cellulose acetate functional groups synthesized from corn husk (Zea mays). In Proceedings of the IOP Conference Series: Earth and Environmental Science, West Bengal, India, 16–18 August 2021; p. 012060. [Google Scholar]
- Vetrivel, S.; Saraswathi, M.S.A.; Rana, D.; Nagendran, A. Fabrication of cellulose acetate nanocomposite membranes using 2D layered nanomaterials for macromolecular separation. Int. J. Biol. Macromol. 2018, 107, 1607–1612. [Google Scholar] [CrossRef]
- Chen, X.; Kuo, D.-H.; Hou, Y.-X. Enhancing the photodegradation of charged pollutants under visible light in Ag2O/g-C3N4 catalyst by Coulombic interaction. J. Mater. Sci. 2017, 52, 5147–5154. [Google Scholar] [CrossRef]
- Ibrahim, R.M.; Markom, M.; Abdullah, H. Optical Properties of Ni 2+ -, Co 2+ -, and Mn 2+ -doped ZnS Nanoparticles Synthesized Using Reverse Micelle Method. ECS J. Solid State Sci. Technol. 2015, 4, 31–37. [Google Scholar] [CrossRef]
- Ashraf, S.; El-Morsy, M.; Awwad, N.S.; Ibrahium, H.A. Physicochemical changes of hydroxyapatite, V2O5, and graphene oxide composites for medical usages. J. Aust. Ceram. Soc. 2022, 58, 1399–1413. [Google Scholar] [CrossRef]
- Gul, S.; Rehan, Z.A.; Khan, S.A.; Akhtar, K.; Khan, M.A.; Khan, M.; Rashid, M.I.; Asiri, A.M.; Khan, S.B. Antibacterial PES-CA-Ag2O nanocomposite supported Cu nanoparticles membrane toward ultrafiltration, BSA rejection and reduction of nitrophenol. J. Mol. Liq. 2017, 230, 616–624. [Google Scholar] [CrossRef]
- Rezaee, R.; Nasseri, S.; Mahvi, A.; Jafari, A.; Safari, M.; Shahmoradi, B.; Alimohammadi, M.; Khazaei, M.; Maroosi, M. Fabrication of ultrathin graphene oxide-coated membrane with hydrophilic properties for arsenate removal from water. J. Adv. Environ. Health Res. 2016, 4, 169. [Google Scholar] [CrossRef]
- Ashraf, S.; Ahmed, M.; Ibrahium, H.A.; Awwad, N.S.; Abdel-Fattah, E.; Ghoniem, M. Nanofibers of polycaprolactone containing hydroxyapatite doped with aluminum/vanadate ions for wound healing applications. New J. Chem. 2021, 45, 22610–22620. [Google Scholar] [CrossRef]
- Shah, A.; Haq, S.; Rehman, W.; Waseem, M.; Shoukat, S.; Rehman, M.-u. Photocatalytic and antibacterial activities of paeonia emodi mediated silver oxide nanoparticles. Mater. Res. Express 2019, 6, 045045. [Google Scholar] [CrossRef]
- Sarraf, M.; Dabbagh, A.; Razak, B.A.; Nasiri-Tabrizi, B.; Hosseini, H.R.M.; Saber-Samandari, S.; Kasim, N.H.A.; Yean, L.K.; Sukiman, N.L. Silver oxide nanoparticles-decorated tantala nanotubes for enhanced antibacterial activity and osseointegration of Ti6Al4V. Mater. Des. 2018, 154, 28–40. [Google Scholar] [CrossRef]
- Gabrielyan, L.; Badalyan, H.; Gevorgyan, V.; Trchounian, A. Comparable antibacterial effects and action mechanisms of silver and iron oxide nanoparticles on Escherichia coli and Salmonella typhimurium. Sci. Rep. 2020, 10, 13145. [Google Scholar] [CrossRef]








| CA (cm−1) | Ag2O/CA (cm−1) | ZnS/CA (cm−1) | Ag2O/ZnS/CA (cm−1) | Ag2O/ZnS/GO/CA (cm−1) | Assignment | Refs |
|---|---|---|---|---|---|---|
| - | - | 485 | 485.2 | 457.6 | Zn-S | [43] |
| - | 561.8 | - | 561.9 | 562 | Ag-O | [42] |
| - | - | 677.2 | 671.5 | 676 | Zn-S | [43] |
| 901.69 | 901.03 | 901.33 | 901.25 | 901.38 | C-H | [39] |
| 1027.16 | 1027.17 | 1026.90 | 1027.53 | 1027.30 | C-O Zn-S | [36,43] |
| 1211.88 | 1212.74 | 1212.55 | 1212.36 | 1212.02 | C-O | [36] |
| 1368.24 | 1367.76 | 1368.30 | 1368.11 | 1367.86 | C-H | [37] |
| - | 1380.2 | - | 1378.2 | 1377.9 | Ag-O | [42] |
| 1663.46 | 1662.89 | 1662.58 | 1663.23 | 1663.25 | C=C | [37] |
| 1738.44 | 1743.94 | 1746.07 | 1744.29 | 1746.30 | C=O | [37] |
| 2901.12 | 2922.70 | 2912.33 | 2922.70 | 2931.48 | C-H | [44] |
| 3431.03 | 3451.77 | 3462.15 | 3431.03 | 3422.25 | O-H | [36] |
| Element | Atomic (%) |
|---|---|
| C K | 70.78 |
| N K | 4.19 |
| O K | 23.43 |
| S K | 0.41 |
| AgL | 0.81 |
| ZnK | 0.38 |
| Sample | Angle | Stander Deviation |
|---|---|---|
| CA | 48.04 | 1.59806 |
| Ag2O/CA | 46.12 | 2.65872 |
| ZnS/CA | 45.64 | 2.50316 |
| Ag2O/ZnS/CA | 26.285 | 2.12839 |
| Ag2O/ZnS/GO/CA | 34.045 | 3.24562 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharthi, A.F.; Gouda, M.; Khalaf, M.M.; Elmushyakhi, A.; Abou Taleb, M.F.; Abd El-Lateef, H.M. Cellulose-Acetate-Based Films Modified with Ag2O and ZnS as Nanocomposites for Highly Controlling Biological Behavior for Wound Healing Applications. Materials 2023, 16, 777. https://doi.org/10.3390/ma16020777
Alharthi AF, Gouda M, Khalaf MM, Elmushyakhi A, Abou Taleb MF, Abd El-Lateef HM. Cellulose-Acetate-Based Films Modified with Ag2O and ZnS as Nanocomposites for Highly Controlling Biological Behavior for Wound Healing Applications. Materials. 2023; 16(2):777. https://doi.org/10.3390/ma16020777
Chicago/Turabian StyleAlharthi, Amjad F., Mohamed Gouda, Mai M. Khalaf, Abraham Elmushyakhi, Manal F. Abou Taleb, and Hany M. Abd El-Lateef. 2023. "Cellulose-Acetate-Based Films Modified with Ag2O and ZnS as Nanocomposites for Highly Controlling Biological Behavior for Wound Healing Applications" Materials 16, no. 2: 777. https://doi.org/10.3390/ma16020777
APA StyleAlharthi, A. F., Gouda, M., Khalaf, M. M., Elmushyakhi, A., Abou Taleb, M. F., & Abd El-Lateef, H. M. (2023). Cellulose-Acetate-Based Films Modified with Ag2O and ZnS as Nanocomposites for Highly Controlling Biological Behavior for Wound Healing Applications. Materials, 16(2), 777. https://doi.org/10.3390/ma16020777