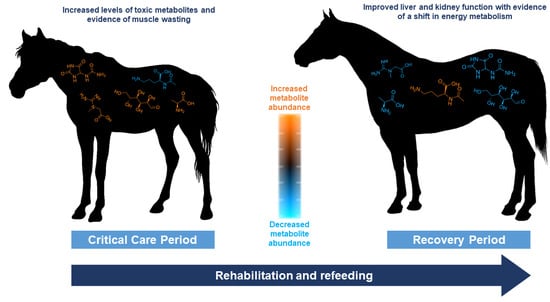
Metabolomic Profiles in Starved Light Breed Horses during the Refeeding Process
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Equine Enrollment
2.2. Study Timeline and Sample Collection
2.3. Diet Formulation
2.4. Blood Chemistry Analysis
2.5. Metabolomics Extractions
2.6. Ultrahigh Performance Liquid Chromatography Mass Spectrometry High Resolution Mass Spectrometry (UHPLC-HRMS)
2.7. Data Analysis
3. Results
3.1. Equine Refeeding Outcomes
3.2. Analysis of Blood Chemistry Panels
3.3. Fold Change Analysis of Metabolites Detected in Equine Subjects during the CCP and RPs
3.4. Differences of the Metabolic Profiles of Equine Subjects in the CCP vs. the RP
3.5. Effects of Subject-Specific Attributes on the Metabolome
4. Discussion
4.1. Animal Origin and Source
4.2. Blood Chemistry Analysis Suggests Improved Biological Function during the RP
4.3. Potentially Toxic Metabolites and Indicators of Oxidative Stress Were in Lower Abundance during the RP
4.4. Metabolites Regulated by Skeletal Muscle Were Altered between the CCP and RP
4.5. Significant Differences Were Observed in Metabolites Related to Liver and Kidney Function during the CCP and RPs
4.6. The Plasma Metabolome Illuminates Differences in Equine Subject-Specific Attributes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, L.G.; Self, A.C.; Hines, M.T.; Ivey, J.L.Z. Clinical Factors Associated with Survival Outcomes in Starved Equids: A Retrospective Case Series. J. Equine Vet.-Sci. 2021, 101, 103370. [Google Scholar] [CrossRef] [PubMed]
- Lenz, T.R. The Unwanted Horse in the United States: An Overview of the Issue. J. Equine Vet.- Sci. 2009, 29, 253–258. [Google Scholar] [CrossRef]
- Stull, C.; Hullinger, P.; Rodiek, A. Fat Supplementation to Alfalfa Diets for Refeeding the Starved Horse. Prof. Anim. Sci. 2003, 19, 47–54. [Google Scholar] [CrossRef]
- Becvarova, I.; Pleasant, R.S.; Thatcher, C.D. Clinical Assessment of Nutritional Status and Feeding Programs in Horses. Vet.-Clin. N. Am. Equine Pract. 2009, 25, 1–21. [Google Scholar] [CrossRef]
- Merriam-Webster. Available online: https://www.merriam-webster.com/dictionary/malnutrition#:%7E:text=%3A%20faulty%20nutrition%20due%20to%20inadequate,Sentences%20Learn%20More%20about%20malnutrition (accessed on 21 May 2022).
- Finn, P.F.; Dice, J.F. Proteolytic and lipolytic responses to starvation. Nutrition 2006, 22, 830–844. [Google Scholar] [CrossRef]
- Henneke, D.R.; Potter, G.D.; Kreider, J.L.; Yeates, B.F. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 1983, 15, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry-based metabolomics. Mass Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef]
- Jiang, P.; Stanstrup, J.; Thymann, T.; Sangild, P.T.; Dragsted, L.O. Progressive Changes in the Plasma Metabolome during Malnutrition in Juvenile Pigs. J. Proteome Res. 2016, 15, 447–456. [Google Scholar] [CrossRef]
- Beachler, T.M.; Gracz, D.R.M.; Bailey, B.A.S.; Borst, L.; Ellis, E.K.; Dollen, V.A.K.; Lyle, K.S.; Nebel, A.; Andrews, C.N.; Koipalli, J. Plasma metabolomic profiling of healthy pregnant mares and mares with experimentally induced placentitis. Equine Vet. J. 2021, 53, 85–93. [Google Scholar] [CrossRef]
- Escalona, E.E.; Leng, J.; Dona, A.C.; Marrifield, C.A.; Holmes, E.; Proudman, C.J.; Swann, J.R. Dominant components of the Thoroughbred metabolome characterised by 1H-nuclear magnetic resonance spectroscopy: A metabolite atlas of common biofluids. Equine Vet. J. 2015, 47, 721–730. [Google Scholar] [CrossRef] [Green Version]
- Keen, B.; Cawley, A.; Reedy, B.; Fu, S. Metabolomics in clinical and forensic toxicology, sports anti-doping and veterinary residues. Drug Test. Anal. 2022, 14, 794–807. [Google Scholar] [CrossRef]
- Klein, D.J.; Anthony, T.G.; McKeever, K.H. Metabolomics in equine sport and exercise. J. Anim. Physiol. Anim. Nutr. 2020, 105, 140–148. [Google Scholar] [CrossRef]
- Freemark, M. Metabolomics in Nutrition Research: Biomarkers Predicting Mortality in Children with Severe Acute Malnutrition. Food Nutr. Bull. 2015, 36 (Suppl. S1), S88–S92. [Google Scholar] [CrossRef]
- Yang, Q.-J.; Zhao, J.-R.; Hao, J.; Li, B.; Huo, Y.; Han, Y.-L.; Wan, L.-L.; Li, J.; Huang, J.; Lu, J.; et al. Serum and urine metabolomics study reveals a distinct diagnostic model for cancer cachexia. J. Cachexia Sarcopenia Muscle 2018, 9, 71–85. [Google Scholar] [CrossRef]
- Heusner, G. Ad libitum feeding of mature horses to achieve rapid weight gain. In Proceedings of the 13th Equine Nutrition Physiology Symposium, Gainesville, FL, USA, 21–23 January 1993. [Google Scholar]
- Council, N.R. Nutrient Requirements of Horses, 6th ed.; National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Weaver, K.; Feldman, M.; Stewart, C.; Thayer, T. Metabolic responses of chronically starved horses to refeeding with three isoenergetic diets. J. Am. Vet. Med. Assoc. 1998, 212, 691–696. [Google Scholar]
- Rabinowitz, J.D.; Kimball, E. Acidic Acetonitrile for Cellular Metabolome Extraction from Escherichia coli. Anal. Chem. 2007, 79, 6167–6173. [Google Scholar] [CrossRef]
- Bazurto, J.V.; Dearth, P.S.; Tague, D.E.; Campagna, R.S.; Downs, M.D. Untargeted metabolomics confirms and extends the understanding of the impact of aminoimidazole carboxamide ribotide (AICAR) in the metabolic network of Salmonella enterica. Microb. Cell 2017, 5, 74–87. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Worley, B.; Halouska, S.; Powers, R. Utilities for quantifying separation in PCA/PLS-DA scores plots. Anal. Biochem. 2013, 433, 102–104. [Google Scholar] [CrossRef]
- Pusterla, N.; Barnum, S.; Miller, J.; Varnell, S.; Dallap-Schaer, B.; Aceto, H.; Simeone, A. Investigation of an EHV-1 Outbreak in the United States Caused by a New H752 Genotype. Pathogens 2021, 10, 747. [Google Scholar] [CrossRef]
- Fleurance, G.R.; Duncan, P.; Mallevaud, B. Daily intake and the selection of feeding sites by horses in heterogeneous wet grasslands. Anim. Res. 2001, 50, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Saastamoinen, M.; Särkijärvi, S.; Suomala, H. Protein Source and Intake Effects on Diet Digestibility and N Excretion in Horses—A Risk of Environmental N Load of Horses. Animals 2021, 11, 3568. [Google Scholar] [CrossRef]
- O’Connor, C.I.; Nielsen, B.D.; Woodward, A.D.; Spooner, H.S.; Ventura, B.A.; Turner, K.K. Mineral balance in horses fed two supplemental silicon sources. J. Anim. Physiol. Anim. Nutr. 2008, 92, 173–181. [Google Scholar] [CrossRef]
- Parraga, M.E.; Carlson, G.P.; Thurmond, M. Serum protein concentrations in horses with severe liver disease: A retrospective study and review of the literature. J. Vet. Intern. Med. 1995, 9, 154–161. [Google Scholar] [CrossRef]
- Urayama, S.; Arima, D.; Mizobe, F.; Shinzaki, Y.; Nomura, M.; Minamijima, Y.; Kusano, K. Blood glucose is unlikely to be a prognostic biomarker in acute colitis with systemic inflammatory response syndrome in Thoroughbred racehorses. J. Equine Sci. 2018, 29, 15–19. [Google Scholar] [CrossRef]
- Hollis, A.R.; Boston, R.C.; Corley, K.T. Blood glucose in horses with acute abdominal disease. J. Vet. Intern. Med. 2007, 21, 1099–1103. [Google Scholar] [CrossRef]
- Toribio, R.E. Essentials of Equine Renal and Urinary Tract Physiology. Vet. Clin. N. Am. Equine Pract. 2007, 23, 533–561. [Google Scholar] [CrossRef]
- Doll, S.K.; Haimerl, P.; Bartel, A.; Arlt, S.P. Determination of reference intervals for nonesterified fatty acids in the blood serum of healthy dogs. Vet.-Rec. Open 2022, 9, e40. [Google Scholar] [CrossRef]
- Farhana, A.; Lappin, S.L. Biochemistry, Lactate Dehydrogenase; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Zoppini, G.; Cacciatori, V.; Negri, C.; Stoico, V.; Lippi, G.; Targher, G.; Bonora, E. The aspartate aminotransferase-to-alanine aminotransferase ratio predicts all-cause and cardiovascular mortality in patients with type 2 diabetes. Medicine 2016, 95, e4821. [Google Scholar] [CrossRef]
- Klein, R.; Nagy, O.; Tóthová, C.; Chovanová, F. Clinical and Diagnostic Significance of Lactate Dehydrogenase and Its Isoenzymes in Animals. Vet.-Med. Int. 2020, 2020, 5346483. [Google Scholar] [CrossRef]
- Ono, T.; Yamada, Y.; Hata, A.; Miyama, T.S.; Shibano, K.; Iwata, E.; Ohzawa, E.; Kitagawa, H. Reference values of hematological and blood biochemical parameters for the Noma horse. J. Equine Sci. 2019, 30, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Fielder, S.E. Serum Biochemical Reference Ranges. 2015. Available online: https://www.merckvetmanual.com/special-subjects/reference-guides/serum-biochemical-reference-ranges (accessed on 12 September 2022).
- Jang, C.; Chen, L.; Rabinowitz, J.D. Metabolomics and Isotope Tracing. Cell 2018, 173, 822–837. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Goldansaz, S.A.; Guo, A.C.; Sajed, T.; Steele, M.A.; Plastow, G.S.; Wishart, D.S. Livestock metabolomics and the livestock metabolome: A systematic review. PLoS ONE 2017, 12, e0177675. [Google Scholar] [CrossRef]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 29–34. [Google Scholar] [CrossRef]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef]
- Ghone, R.A. A Study of Oxidative Stress Biomarkers and Effect of Oral Antioxidant Supplementation in Severe Acute Malnutrition. J. Clin. Diagn. Res. 2013, 7, 2146–2148. [Google Scholar] [CrossRef]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef]
- Foroutan, A.; Fitzsimmons, C.; Mandal, R.; Piri-Moghadam, H.; Zheng, J.; Guo, A.; Li, C.; Guan, L.L.; Wishart, D.S. The Bovine Metabolome. Metabolites 2020, 10, 233. [Google Scholar] [CrossRef]
- Parenti, M.; McClorry, S.; Maga, E.A.; Slupsky, C.M. Metabolomic changes in severe acute malnutrition suggest hepatic oxidative stress: A secondary analysis. Nutr. Res. 2021, 91, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Schuback, K.; Essén-Gustavsson, B.; Persson, S.G.B. Effect of creatine supplementation on muscle metabolic response to a maximal treadmill exercise test in Standardbred horses. Equine Vet.-J. 2010, 32, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Bergero, D.; Assenza, A.; Schiavone, A.; Piccione, G.; Perona, G.; Caola, G. Amino acid concentrations in blood serum of horses performing long lasting low-intensity exercise. J. Anim. Physiol. Anim. Nutr. 2005, 89, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Qiu, L.; Xiao, Q.; Wang, Y.; Meng, X.; Xu, R.; Wang, S.; Na, R. Obesity and diabetes related plasma amino acid alterations. Clin. Biochem. 2013, 46, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Sarabhai, T.; Roden, M. Hungry for your alanine: When liver depends on muscle proteolysis. J. Clin. Investig. 2019, 129, 4563–4566. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Aw, W.; Kaneko, K. Metabolic Interactions of Purine Derivatives with Human ABC Transporter ABCG2: Genetic Testing to Assess Gout Risk. Pharmaceuticals 2013, 6, 1347–1360. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Xie, C.; Fang, J. Uridine Metabolism and Its Role in Glucose, Lipid, and Amino Acid Homeostasis. BioMed Res. Int. 2020, 2020, 7091718. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Børsheim, E.; Wolfe, R.R. Potential Ergogenic Effects of Arginine and Creatine Supplementation. J. Nutr. 2004, 134 (Suppl. S10), 2888S–2894S. [Google Scholar] [CrossRef]
- Klein, C.J.; Stanek, G.S.; Wiles, C.E. Overfeeding Macronutrients to Critically Ill Adults: Metabolic Complications. J. Am. Diet. Assoc. 1998, 98, 795–806. [Google Scholar] [CrossRef]
- McKeever, K.H. Aging and how it affects the physiological response to exercise in the horse. Clin. Tech. Equine Pract. 2003, 2, 258–265. [Google Scholar] [CrossRef]




| Subject Identifier | Sex | Breed | Approximate Age (Years) |
|---|---|---|---|
| A | Mare | Quarter Horse | 19 |
| A2 | Mare | Tennessee Walking Horse | 22 |
| B | Mare | Appendix | 20 |
| C | Mare | Quarter Horse | 20 |
| D | Mare | Tennessee Walking Horse | 20 |
| E | Mare | Quarter Horse/Arabian | 18 |
| F | Gelding | Appendix | 16 |
| G | Gelding | Appendix | 23 |
| H | Mare | Standardbred | 15 |
| I | Gelding | Quarter Horse | 17 |
| Nutrient | Timothy Hay | Equine Senior | Free Balance Mineral |
|---|---|---|---|
| Digestible Energy (mCal/kg) | 1.95 | 2.7 | 0 |
| Protein (%) | 9.88 | 15.5 | 1.55 |
| Lysine (%) | 0.34 | 0.7 | N/A |
| Fat (%) | 1.29 | 6.2 | 0.1 |
| Acid Detergent Fiber (%) | 39.50 | 22.54 | N/A |
| Neutral Detergent Fiber (%) | 62.23 | 38.89 | N/A |
| Calcium (%) | 0.21 | 0.77 | 13.52 |
| Phosphate (%) | 0.27 | 0.55 | 12.66 |
| Potassium (%) | 2.39 | 1.6 | 0.66 |
| Analyte | Abbreviation | Units | Matrix |
|---|---|---|---|
| Lactate dehydrogenase | LDH | U/L | Plasma/serum |
| Alkaline phosphatase | ALP | U/L | Plasma/serum |
| Albumin | ALB | g/dL | Plasma/serum |
| Blood urea nitrogen | BUN | mg/dL | Plasma/serum |
| Gamma-glutamyl transferase | GGT | U/L | Plasma/serum |
| Aspartate amino transferase | AST | U/L | Plasma/serum |
| Glucose | GLUC | mg/dL | Plasma/serum |
| Phosphate | PHOS | mg/dL | Plasma/serum |
| Total protein | TP | g/dL | Plasma/serum |
| Alanine transaminase | ALT | U/L | Plasma/serum |
| Creatinine | CREAT | mg/dL | Plasma/serum |
| Triglycerides | TRIG | mg/dL | Plasma/serum |
| Creatine kinase | CK | U/L | Serum only |
| Non-esterified fatty acids | NEFA | mEq/L | Serum only |
| Serum calcium | SCA | mg/dL | Serum only |
| Subject Identifier | Program Outcome | Samples Taken | |
|---|---|---|---|
| CCP | RP | ||
| A | Did not complete | 5 | NA |
| A2 | Did not complete | 7 | NA |
| B | Did not complete | 7 | NA |
| C | Completed | 7 | 4 |
| D | Did not complete | 7 | 1 |
| E | Completed | 7 | 4 |
| F | Completed | 7 | 3 |
| G | Completed | 7 | 3 |
| H | Completed | 7 | 3 |
| I | Completed | 7 | 2 |
| Metabolite Class | Significantly Increased or Decreased (RP/CCP) | Metabolite | Biological Significance 1 |
|---|---|---|---|
| Amino acids (metabolism, precursors, derivatives) | Decreased | Glutamine |
|
| Decreased | Methionine |
| |
| Increased | N-acetylornithine |
| |
| Decreased | Phenylalanine |
| |
| Decreased 2 | Alanine |
| |
| Sarcosine |
| ||
| Decreased | Creatine |
| |
| Nucleosides/nucleotides | Decreased | Uridine |
|
| Unclassified metabolites | Decreased | Uric acid |
|
| Decreased | Allantoin |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Main, S.C.; Brown, L.P.; Melvin, K.R.; Campagna, S.R.; Voy, B.H.; Castro, H.F.; Strickland, L.G.; Hines, M.T.; Jacobs, R.D.; Gordon, M.E.; et al. Metabolomic Profiles in Starved Light Breed Horses during the Refeeding Process. Animals 2022, 12, 2527. https://doi.org/10.3390/ani12192527
Main SC, Brown LP, Melvin KR, Campagna SR, Voy BH, Castro HF, Strickland LG, Hines MT, Jacobs RD, Gordon ME, et al. Metabolomic Profiles in Starved Light Breed Horses during the Refeeding Process. Animals. 2022; 12(19):2527. https://doi.org/10.3390/ani12192527
Chicago/Turabian StyleMain, Sawyer C., Lindsay P. Brown, Kelly R. Melvin, Shawn R. Campagna, Brynn H. Voy, Hector F. Castro, Lewrell G. Strickland, Melissa T. Hines, Robert D. Jacobs, Mary E. Gordon, and et al. 2022. "Metabolomic Profiles in Starved Light Breed Horses during the Refeeding Process" Animals 12, no. 19: 2527. https://doi.org/10.3390/ani12192527
APA StyleMain, S. C., Brown, L. P., Melvin, K. R., Campagna, S. R., Voy, B. H., Castro, H. F., Strickland, L. G., Hines, M. T., Jacobs, R. D., Gordon, M. E., & Ivey, J. L. Z. (2022). Metabolomic Profiles in Starved Light Breed Horses during the Refeeding Process. Animals, 12(19), 2527. https://doi.org/10.3390/ani12192527