Voltammetric Detection of Caffeine in Beverages at Nafion/Graphite Nanoplatelets Layer-by-Layer Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Apparatus
2.3. Preparation of Nafion/GNPs Composite Solutions
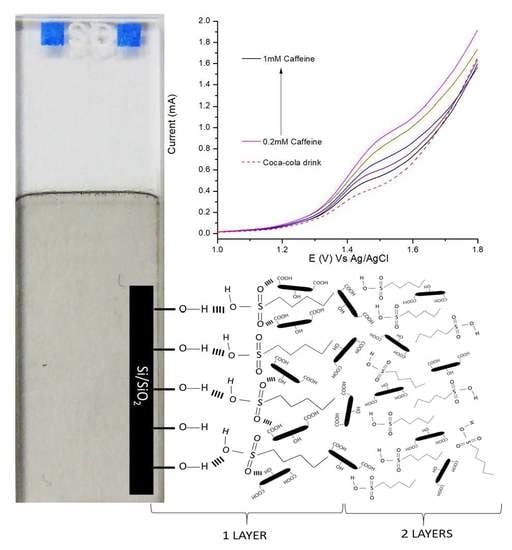
2.4. Preparation of Nafion/GNP LbL Films
2.5. Detection of Caffeine in Real Samples
3. Results
3.1. Morphology and Structure of Multilayer Nafion/GNPs Films
3.2. Effect of Graphite Nanoplatelets and Nafion Concentration in the LbL Film Formation
3.3. Electrochemical Characterization of Multilayer Nafion/GNPs Films
3.4. Detection of Caffeine
3.5. Detection of Caffeine in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pumera, M.; Ambrosi, A.; Bonanni, A.; Chng, E.L.K.; Poh, H.L. Graphene for electrochemical sensing and biosensing. Trac-Trends Anal. Chem. 2010, 29, 954–965. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Voeroes, J.; Reimhult, E. Electrochemical biosensors-sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Bertoncello, P.; D’Souza, F.; Kadish, K.M. Carbon Nanomaterials for Electrochemical Sensing. In Handbook of Carbon Nano Materials; D’Souza, F., Kadish, K.M., Eds.; World Scientific: London, UK, 2016; Volume 8, pp. 225–260. [Google Scholar]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Shen, Z. A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Kosynkin, D.V.; Higginbotham, A.L.; Sinitskii, A.; Lomeda, J.R.; Dimiev, A.; Price, B.K.; Tour, J.M. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 2009, 458, 872. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Khannanov, A.; Vakhitov, I.; Kiiamov, A.; Shukhina, K.; Tour, J.M. Revisiting the Mechanism of Oxidative Unzipping of Multiwall Carbon Nanotubes to Graphene Nanoribbons. ACS Nano 2018, 12, 3985–3993. [Google Scholar] [CrossRef]
- Deokar, G.; Avila, J.; Razado-Colambo, I.; Codron, J.L.; Boyaval, C.; Galopin, E.; Asensio, M.C.; Vignaud, D. Towards high quality CVD graphene growth and transfer. Carbon 2015, 89, 82–92. [Google Scholar] [CrossRef]
- Huang, W.-H.; Lin, C.-H.; Lin, B.-S.; Sun, C.-L. Low-Temperature CVD Graphene Nanostructures on Cu and Their Corrosion Properties. Materials 2018, 11, 1989. [Google Scholar] [CrossRef]
- Gao, X.F.; Jang, J.; Nagase, S. Hydrazine and Thermal Reduction of Graphene Oxide: Reaction Mechanisms, Product Structures, and Reaction Design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Lu, J.; Do, I.; Fukushima, H.; Lee, I.; Drzal, L.T. Stable Aqueous Suspension and Self-Assembly of Graphite Nanoplatelets Coated with Various Polyelectrolytes. J. Nanomater. 2010, 2010, 11. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Denno, M.E.; Pyakurel, P.; Venton, B.J. Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review. Anal. Chim. Acta 2015, 887, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Bertoncello, P.; Ram, M.K.; Notargiacomo, A.; Ugo, P.; Nicolini, C. Fabrication and physico-chemical properties of Nafion Langmuir–Schaefer films. Phys. Chem. Chem. Phys. 2002, 4, 4036–4043. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.-D. Buildup of ultrathin multilayer films by a self-assembly process, 1 consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Makromol. Chem. Macromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Yu, B.; Liu, X.; Cong, H.; Yuan, H.; Wang, D.; Li, Z. Graphene-Based Multilayers Constructed from Layer-by-Layer Self-Assembly Techniques. J. Nanosci. Nanotechnol. 2014, 14, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Min, S.H.; Gu, M.; Jung, Y.K.; Lee, W.; Lee, J.U.; Seong, D.G.; Kim, B.-S. Layer-by-Layer Assembly for Graphene-Based Multilayer Nanocomposites: Synthesis and Applications. Chem. Mater. 2015, 27, 3785–3796. [Google Scholar] [CrossRef]
- Nehlig, A.; Daval, J.-L.; Debry, G. Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Rev. 1992, 17, 139–170. [Google Scholar] [CrossRef]
- del Campo, G.; Berregi, I.; Caracena, R.; Zuriarrain, J. Quantitative determination of caffeine, formic acid, trigonelline and 5-(hydroxymethyl) furfural in soluble coffees by 1H NMR spectrometry. Talanta 2010, 81, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhao, Z.; Chen, Y.; Hu, H.; Qiu, J. Low temperature plasma-mediated synthesis of graphene nanosheets for supercapacitor electrodes. J. Mater. Chem. 2012, 22, 6061–6066. [Google Scholar] [CrossRef]
- Baker, J.; McGettrick, J.D.; Gethin, D.T.; Watson, T.M. Impedance Characteristics of Transparent GNP-Pt Ink Catalysts for Flexible Dye Sensitized Solar Cells. J. Electrochem. Soc. 2015, 162, H564–H569. [Google Scholar] [CrossRef]
- Kaciulis, S. Spectroscopy of carbon: From diamond to nitride films. Surf. Interface Anal. 2012, 44, 1155–1161. [Google Scholar] [CrossRef]
- Wu, X.; Sacher, E.; Meunier, M. The effects of hydrogen bonds on the adhesion of inorganic oxide particles on hydrophilic silicon surfaces. J. Appl. Phys. 1999, 86, 1744–1748. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Q.; Hao, Y.; Li, Y.; Fang, Y.; Chen, D. Alternate Multilayer Films of Poly(vinyl alcohol) and Exfoliated Graphene Oxide Fabricated via a Facial Layer-by-Layer Assembly. Macromolecules 2010, 43, 9411–9416. [Google Scholar] [CrossRef]
- Park, J.S.; Cho, S.M.; Kim, W.-J.; Park, J.; Yoo, P.J. Fabrication of Graphene Thin Films Based on Layer-by-Layer Self-Assembly of Functionalized Graphene Nanosheets. ACS Appl. Mater. Interfaces 2011, 3, 360–368. [Google Scholar] [CrossRef]
- Gupta, B.; Kumar, N.; Panda, K.; Kanan, V.; Joshi, S.; Visoly-Fisher, I. Role of oxygen functional groups in reduced graphene oxide for lubrication. Sci. Rep. 2017, 7, 45030. [Google Scholar] [CrossRef]
- Qiao, S.-J.; Xu, X.-N.; Qiu, Y.; Xiao, H.-C.; Zhu, Y.-F. Simultaneous Reduction and Functionalization of Graphene Oxide by 4-Hydrazinobenzenesulfonic Acid for Polymer Nanocomposites. Nanomaterials 2016, 6, 29. [Google Scholar] [CrossRef]
- Drewniak, S.; Muzyka, R.; Stolarczyk, A.; Pustelny, T.; Kotyczka-Morańska, M.; Setkiewicz, M. Studies of Reduced Graphene Oxide and Graphite Oxide in the Aspect of Their Possible Application in Gas Sensors. Sensors 2016, 16, 103. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy; Cengage Learning: Belmont, CA, USA, 2008. [Google Scholar]
- Oh, Y.J.; Yoo, J.J.; Kim, Y.I.; Yoon, J.K.; Yoon, H.N.; Kim, J.-H.; Park, S.B. Oxygen functional groups and electrochemical capacitive behavior of incompletely reduced graphene oxides as a thin-film electrode of supercapacitor. Electrochim. Acta 2014, 116, 118–128. [Google Scholar] [CrossRef]
- Page, A.J.; Chou, C.-P.; Pham, B.Q.; Witek, H.A.; Irle, S.; Morokuma, K. Quantum chemical investigation of epoxide and ether groups in graphene oxide and their vibrational spectra. Phys. Chem. Chem. Phys. 2013, 15, 3725–3735. [Google Scholar] [CrossRef]
- Mohammadi, S.; Afshar Taromi, F.; Shariatpanahi, H.; Neshati, J.; Hemmati, M. Electrochemical and anticorrosion behavior of functionalized graphite nanoplatelets epoxy coating. J. Ind. Eng. Chem. 2014, 20, 4124–4139. [Google Scholar] [CrossRef]
- Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study AU—Tucureanu, Vasilica. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar]
- Zhu, B.; Wang, F.; Li, P.; Wang, C.; Gu, Y. Surface oxygen-containing defects of graphene nanosheets with tunable nonlinear optical absorption and refraction. Phys. Chem. Chem. Phys. 2018, 20, 27105–27114. [Google Scholar] [CrossRef] [PubMed]
- Konkena, B.; Vasudevan, S. Understanding Aqueous Dispersibility of Graphene Oxide and Reduced Graphene Oxide through pKa Measurements. J. Phys. Chem. Lett. 2012, 3, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Kurihara, S.; Tateishi, H.; Hatakeyama, K.; Koinuma, M.; Yokoi, H.; Hara, M.; Ishikawa, H.; Matsumoto, Y. pH-driven, reversible epoxy ring opening/closing in graphene oxide. Carbon 2015, 84, 560–566. [Google Scholar] [CrossRef]
- Bhattacharya, M. Polymer Nanocomposites—A Comparison between Carbon Nanotubes, Graphene, and Clay as Nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef]
- Paul, D.K.; Karan, K.; Docoslis, A.; Giorgi, J.B.; Pearce, J. Characteristics of Self-Assembled Ultrathin Nafion Films. Macromolecules 2013, 46, 3461–3475. [Google Scholar] [CrossRef]
- Rivas, G.A.; Miscoria, S.A.; Desbrieres, J.; Barrera, G.D. New biosensing platforms based on the layer-by-layer self-assembling of polyelectrolytes on Nafion/carbon nanotubes-coated glassy carbon electrodes. Talanta 2007, 71, 270–275. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Y.; Li, C.; Qin, C.; Shi, M.; Ye, M. Layer-by-Layer Self-Assembly of Graphene Nanoplatelets. Langmuir 2009, 25, 6122–6128. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, L.; Wang, Q.; Fan, Y.; Shang, J.; Qiu, S.; Li, J.; Zhang, W.; Wu, X. Supramolecular self-assembly of layer-by-layer graphene film driven by the synergism of π–π and hydrogen bonding interaction. J. Photochem. Photobiol. A Chem. 2018, 355, 249–255. [Google Scholar] [CrossRef]
- Hao, E.; Lian, T. Layer-by-Layer Assembly of CdSe Nanoparticles Based on Hydrogen Bonding. Langmuir 2000, 16, 7879–7881. [Google Scholar] [CrossRef]
- Tuz Johra, F.; Lee, J.; Jung, W.-G. Facile and Safe Graphene Preparation on Solution Based Platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Suroviec, A. Determining Surface Coverage of Self-Assembled Monolayers on Gold Electrodes. Chem. Educ. 2012, 17, 83. [Google Scholar]
- Bard, A.; Faulkner, L. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; Chapter 6; p. 231. [Google Scholar]
- Wang, Y.; Limon-Petersen, J.G.; Compton, R.G. Measurement of the diffusion coefficients of [Ru(NH3)6]3+ and [Ru(NH3)6]2+ in aqueous solution using microelectrode double potential step chronoamperometry. J. Electroanal. Chem. 2011, 652, 13–17. [Google Scholar] [CrossRef]
- Bertoncello, P.; Ugo, P. Preparation and voltammetric characterization of electrodes coated with Langmuir-Schaefer ultrathin films of Nafion®. J. Braz. Chem. Soc. 2003, 14, 517–522. [Google Scholar] [CrossRef]
- Bertoncello, P.; Dennany, L.; Forster, R.J.; Unwin, P.R. Nafion−Tris(2-2′-bipyridyl)ruthenium(II) Ultrathin Langmuir−Schaefer Films: Redox Catalysis and Electrochemiluminescent Properties. Anal. Chem. 2007, 79, 7549–7553. [Google Scholar] [CrossRef]
- Bertoncello, P.; Wilson, N.R.; Unwin, P.R. One-step formation of ultra-thin chemically functionalized redox-active Langmuir–Schaefer Nafion films. Soft Matter 2007, 3, 1300–1307. [Google Scholar] [CrossRef]
- Bertoncello, P.; Ciani, I.; Li, F.; Unwin, P.R. Measurement of Apparent Diffusion Coefficients within Ultrathin Nafion Langmuir−Schaefer Films: Comparison of a Novel Scanning Electrochemical Microscopy Approach with Cyclic Voltammetry. Langmuir 2006, 22, 10380–10388. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Huang, K.-J.; Wei, S.-Y.; Wu, Z.-W.; Ren, F.-P. A graphene-based electrochemical sensor for sensitive determination of caffeine. Colloids Surf. B Biointerfaces 2011, 84, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Maccaferri, G.; Zanardi, C.; Xia, Z.Y.; Kovtun, A.; Liscio, A.; Terzi, F.; Palermo, V.; Seeber, R. Systematic study of the correlation between surface chemistry, conductivity and electrocatalytic properties of graphene oxide nanosheets. Carbon 2017, 120, 165–175. [Google Scholar] [CrossRef]
- Hernandez-Aldave, S.; Kaspar, R.B.; Letterio, M.P.; Tarat, A.; Yan, Y.; Bertoncello, P. Quaternary phosphonium-based (TPQPCl)-ionomer/graphite nanoplatelets composite chemically modified electrodes: A novel platform for sensing applications. J. Mater. Chem. C 2018, 6, 13293–13304. [Google Scholar] [CrossRef]
- Švorc, L.u.; Tomčík, P.; Svítková, J.; Rievaj, M.; Bustin, D. Voltammetric determination of caffeine in beverage samples on bare boron-doped diamond electrode. Food Chem. 2012, 135, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Sulistyaningrum, I.; Utami, M.P.G.; Istiningrum, R.B.; Siregar, I.M. Comparison Between the Calibration and the Standard Addition Methods in Determining Dissolved Lead in Borobudur’s Control Tanks Water by Flame Atomic Absorption Spectrophotometry (F-AAS). Procedia Chem. 2015, 17, 70–74. [Google Scholar] [CrossRef]
- Alpar, N.; Yardım, Y.; Şentürk, Z. Selective and simultaneous determination of total chlorogenic acids, vanillin and caffeine in foods and beverages by adsorptive stripping voltammetry using a cathodically pretreated boron-doped diamond electrode. Sens. Actuators B Chem. 2018, 257, 398–408. [Google Scholar] [CrossRef]
- Staff, J. Caffeine Content in a Can of Coca-Cola | Coca-Cola GB. Available online: https://www.coca-cola.co.uk/stories/the-caffeine-in-your-can (accessed on 24 December 2018).
- Bargains, S. Available online: https://www.starbargains.co.uk/product/power_energy_shot_drink_250ml/1259 (accessed on 24 December 2018).
- Amiri-Aref, M.; Raoof, J.B.; Ojani, R. A highly sensitive electrochemical sensor for simultaneous voltammetric determination of noradrenaline, acetaminophen, xanthine and caffeine based on a flavonoid nanostructured modified glassy carbon electrode. Sens. Actuators B Chem. 2014, 192, 634–641. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, Q.; Yang, B.; Wang, J.; Ye, B. Determination of caffeine content in tea based on poly(safranine T) electroactive film modified electrode. Food Chem. 2011, 129, 1311–1314. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.P.; Guo, W.; Peng, X.D.; Li, M.; Yuan, Z.B. Sensitive Differential Pulse Stripping Voltammetry of Caffeine in Medicines and Cola Using a Sensor Based on Multi-Walled Carbon Nanotubes and Nafion. Int. J. Electrochem. Sci. 2011, 6, 997–1006. [Google Scholar]
- Gao, Y.; Wang, H.; Guo, L. Simultaneous determination of theophylline and caffeine by large mesoporous carbon/Nafion modified electrode. J. Electroanal. Chem. 2013, 706, 7–12. [Google Scholar] [CrossRef]
- Alizadeh, T.; Ganjali, M.R.; Zare, M.; Norouzi, P. Development of a voltammetric sensor based on a molecularly imprinted polymer (MIP) for caffeine measurement. Electrochim. Acta 2010, 55, 1568–1574. [Google Scholar] [CrossRef]
- Arroyo-Gómez, J.J.; Villarroel-Rocha, D.; de Freitas-Araújo, K.C.; Martínez-Huitle, C.A.; Sapag, K. Applicability of activated carbon obtained from peach stone as an electrochemical sensor for detecting caffeine. J. Electroanal. Chem. 2018, 822, 171–176. [Google Scholar] [CrossRef]
- Gao, L.; Yue, R.; Xu, J.; Liu, Z. One-pot Synthesis of Fe2O3/PEDOT/rGO Nanocomposite for Sensitive Determination of Caffeine. Int. J. Electrochem. Sci 2018, 13, 6791–6802. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Li, L.; Hu, P. Nitrogen-doped carbon nanotubes decorated poly (l-Cysteine) as a novel, ultrasensitive electrochemical sensor for simultaneous determination of theophylline and caffeine. Talanta 2018, 178, 449–457. [Google Scholar] [CrossRef]
- Zhang, G.; Fu, H.; Zou, D.; Xiao, R.; Liu, J.; Li, S. Electrochemical Determination of Caffeine in Tea Using a Polydopamine-Gold Nanocomposite. Int. J. Electrochem. Sci. 2017, 12, 11465–11472. [Google Scholar] [CrossRef]
- Ören, T.; Anık, Ü. Voltammetric determination of caffeine by using gold nanoparticle-glassy carbon paste composite electrode. Measurement 2017, 106, 26–30. [Google Scholar] [CrossRef]
- Shu, X.; Bian, F.; Wang, Q.; Qin, X.; Wang, Y. Electrochemical Sensor for Simultaneous Determination of Theophylline and Caffeine Based on a Novel poly (folic acid)/graphene Composite Film Modified Electrode. Int. J. Electrochem. Sci. 2017, 12, 4251–4264. [Google Scholar] [CrossRef]
- Zhang, Y.; Shang, J.; Jiang, B.; Zhou, X.; Wang, J. Electrochemical Determination of Caffeine in Oolong Tea Based on Polyelectrolyte Functionalized Multi-Walled Carbon Nanotube. Int. J. Electrochem. Sci. 2017, 12, 2552–2562. [Google Scholar] [CrossRef]












| No. of Layers | Φ/(10−5) cm | Surface Coverage (Γ)/(10−9) mol/cm2 | Cp/(mol dm−3) | Dapp/(10−10 cm2·s−1 ) |
|---|---|---|---|---|
| 5 | 3 ± 1.7 | 1.65 ± 0.2 | 0.055 ± 0.02 | 1.06 ± 0.9 |
| 10 | 5 ± 1.9 | 2.80 ± 0.3 | 0.056 ± 0.02 | 1.47 ± 1.1 |
| Electrochemical Technique | Linear Range (µM) | LOD (µM) | Reference | |
|---|---|---|---|---|
| Functionalized MWCNT/GCE | DPV | 10.0‒100.0 | 3.54 | [62] |
| Nafion/Gr/GCE | DPV | 0.4‒40.0 and 40‒600 | 0.12 | [54] |
| PST/Nafion/GCE | LSV | 0.3‒100.0 | 0.10 | [63] |
| MWCNT-Nafion/GCE | DPV | 2.945‒377.0 | 0.513 | [64] |
| Large mesoporous carbon/Nafion/GCE | DPV | 1.3‒230.0 | 0.47 | [65] |
| MIP/Carbon Paste | DPV | 0.06‒25.0 | 0.02 | [66] |
| CA-Zn/GCE | DPV | 39.8‒458 | 28.5 | [67] |
| Fe2O3/PEDOT/rGO/GCE | DPV | 1‒800 | 0.33 | [68] |
| PLCY/N-CNT/GCE | DPV | 0.4–140.0 | 0.02 | [69] |
| PDA/AuNPs/GCE | DPV | 100‒7500 | 0.79 | [70] |
| AuNP-GCPE | DPV | 25–150 and 200–1000 | 0.96 | [71] |
| Poly(FA)/ GR /GCE | DPV | 1‒160 | 0.08 | [72] |
| Nafion/PDDA-MWCNT/GCE | DPV | 0.3–80 | 0.05 | [73] |
| Nafion/GNPs/LbL film | CV | 50–5000 | 24 | This work |
| Nafion/GNPs/LbL film | DPV | 20‒250 | 0.03 | This work |
| Sample | Caffeine (mM)/(mg/mL) | |
|---|---|---|
| Nafion/GNPs | UV-vis Spectroscopic 1 | |
| Coca-Cola | 0.453 mM/0.0879 g·L−1 | 0.45 mM/0.088 g·L−1 |
| Energy drink | 1.97 mM/0.38 g·L−1 | 1.90 mM/0.37 g·L−1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Aldave, S.; Tarat, A.; McGettrick, J.D.; Bertoncello, P. Voltammetric Detection of Caffeine in Beverages at Nafion/Graphite Nanoplatelets Layer-by-Layer Films. Nanomaterials 2019, 9, 221. https://doi.org/10.3390/nano9020221
Hernandez-Aldave S, Tarat A, McGettrick JD, Bertoncello P. Voltammetric Detection of Caffeine in Beverages at Nafion/Graphite Nanoplatelets Layer-by-Layer Films. Nanomaterials. 2019; 9(2):221. https://doi.org/10.3390/nano9020221
Chicago/Turabian StyleHernandez-Aldave, Sandra, Afshin Tarat, James D. McGettrick, and Paolo Bertoncello. 2019. "Voltammetric Detection of Caffeine in Beverages at Nafion/Graphite Nanoplatelets Layer-by-Layer Films" Nanomaterials 9, no. 2: 221. https://doi.org/10.3390/nano9020221
APA StyleHernandez-Aldave, S., Tarat, A., McGettrick, J. D., & Bertoncello, P. (2019). Voltammetric Detection of Caffeine in Beverages at Nafion/Graphite Nanoplatelets Layer-by-Layer Films. Nanomaterials, 9(2), 221. https://doi.org/10.3390/nano9020221