Molecular Mechanisms of Obesity, Diabetes, Inflammation and Aging
Share This Topical Collection
Editors
Topical Collection Information
Dear Colleagues,
Obesity and diabetes have become epidemic worldwide, and the incidences of these chronic metabolic diseases are more prominent in older people. Low-grade chronic inflammation is now recognized as the culprit of both obesity and aging. Inflammation is a hallmark of aging, and many age-associated diseases are associated with inflammation. Thus, the term “inflamm-aging” has been coined.
While tremendous progress has been made in obesity, diabetes, inflammation, and aging over the years, many unanswered questions remain regarding the in-depth molecular mechanisms of these pathological conditions. Immunometabolism has recently merged as a new frontier of biological research, which underscores the significance of immunity in health/disease and the interconnection of different disciplines. The COVID-19 pandemic has further brought immunity to the forefront of science. We now recognize that for inflammatory diseases such as COVID-19, it is not only important to study the initiation of infection/inflammation, but also to understand the resolution of inflammation that is critical for disease resilience and prognosis.
This Topical Collection aims to serve as a catalyst for bringing together like-minded experts to share novel findings and perspectives in “Molecular Mechanisms of Obesity, Diabetes, Inflammation and Aging”. This collection welcomes original research articles as well as up-to-date reviews.
We look forward to reading your contributions.
You may choose our Joint Topical Collection in Geriatrics.
Dr. Yuxiang Sun
Dr. Susanne Talcott
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Biomolecules is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- obesity
- diabetes
- inflammation
- aging
- immunometabolism
- immunity
- microbiome
- nutrition
- stress
- metabolism
Published Papers (19 papers)
Open AccessArticle
Metformin Restrains the Proliferation of CD4+ T Lymphocytes by Inducing Cell Cycle Arrest in Normo- and Hyperglycemic Conditions
by
Ricardo Cartes-Velásquez, Agustín Vera, Bárbara Antilef, Sergio Sanhueza, Liliana Lamperti, Marcelo González-Ortiz and Estefanía Nova-Lamperti
Viewed by 1385
Abstract
CD4+ T lymphocytes play a key role in the modulation of the immune response by orchestrating both effector and regulatory functions. The effect of metformin on the immunometabolism of CD4+ T lymphocytes has been scarcely studied, and its impact under high glucose conditions,
[...] Read more.
CD4+ T lymphocytes play a key role in the modulation of the immune response by orchestrating both effector and regulatory functions. The effect of metformin on the immunometabolism of CD4+ T lymphocytes has been scarcely studied, and its impact under high glucose conditions, particularly concerning effector responses and glucose metabolism, remains unknown. This study aims to evaluate the effect of metformin on the modulation of the effector functions and glucose metabolism of CD4+ T lymphocytes under normo- and hyperglycemic conditions. CD4+ T lymphocytes, obtained from peripheral blood from healthy volunteers, were anti-CD3/CD28-activated and cultured for 4 days with three concentrations of metformin (0.1 mM, 1 mM, and 5 mM) under normoglycemic (5.5 mM) and hyperglycemic (25 mM) conditions. Effector functions such as proliferation, cell count, cell cycle analysis, activation markers and cytokine secretion were analyzed by flow cytometry. Glucose uptake was determined using the 2-NBDG assay, and levels of glucose, lactate, and phosphofructokinase (PFK) activity were assessed by colorimetric assays. Metformin at 5 mM restrained the cell counts and proliferation of CD4+ T lymphocytes by arresting the cell cycle in the S/G2 phase at the beginning of the cell culture, without affecting cell activation, cytokine production, and glucose metabolism. In fact, CD69 expression and IL4 secretion by CD4+ T lymphocytes was higher in the presence of 5 mM than the untreated cells in both glucose conditions. Overall, metformin inhibited proliferation through mechanisms associated with cell cycle arrest, leading to an increase in the S/G2 phases at the expense of G1 in activated CD4+ T lymphocytes in normo- and hyperglycemic conditions. Despite the cell cycle arrest, activated CD4+ T lymphocytes remained metabolically, functionally, and phenotypically activated.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceReview
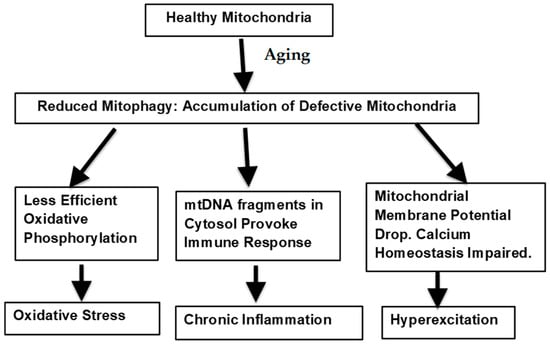
Mitochondrial Dysfunction as the Major Basis of Brain Aging
by
Stephen C. Bondy
Cited by 5 | Viewed by 2366
Abstract
The changes in the properties of three biological events that occur with cerebral aging are discussed. These adverse changes already begin to develop early in mid-life and gradually become more pronounced with senescence. Essentially, they are reflections of the progressive decline in effectiveness
[...] Read more.
The changes in the properties of three biological events that occur with cerebral aging are discussed. These adverse changes already begin to develop early in mid-life and gradually become more pronounced with senescence. Essentially, they are reflections of the progressive decline in effectiveness of key processes, resulting in the deviation of essential biochemical trajectories to ineffective and ultimately harmful variants of these programs. The emphasis of this review is the major role played by the mitochondria in the transition of these three important processes toward more deleterious variants as brain aging proceeds. The immune system: the shift away from an efficient immune response to a more unfocused, continuing inflammatory condition. Such a state is both ineffective and harmful. Reactive oxygen species are important intracellular signaling systems. Additionally, microglial phagocytic activity utilizing short lived reactive oxygen species contribute to the removal of aberrant or dead cells and bacteria. These processes are transformed into an excessive, untargeted, and persistent generation of pro-oxidant free radicals (oxidative stress). The normal efficient neural transmission is modified to a state of undirected, chronic low-level excitatory activity. Each of these changes is characterized by the occurrence of continuous activity that is inefficient and diffused. The signal/noise ratio of several critical biological events is thus reduced as beneficial responses are gradually replaced by their impaired and deleterious variants.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceArticle
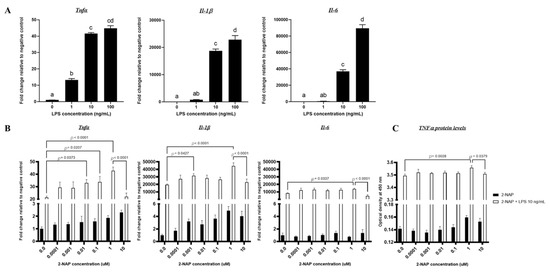
Chronic Exposure to Low-Molecular-Weight Polycyclic Aromatic Hydrocarbons Promotes Lipid Accumulation and Metabolic Inflammation
by
Asia Bright, Fenfen Li, Miranda Movahed, Hang Shi and Bingzhong Xue
Cited by 21 | Viewed by 2601
Abstract
2-naphthol is a low-molecular-weight (LMW) polycyclic aromatic hydrocarbon (PAH) and air pollutant associated with childhood obesity. There has been a recent emergence of studies on the consequences of PAHs on human health. Current epidemiological reports suggest LMW-PAHs may contribute to obesity incidences in
[...] Read more.
2-naphthol is a low-molecular-weight (LMW) polycyclic aromatic hydrocarbon (PAH) and air pollutant associated with childhood obesity. There has been a recent emergence of studies on the consequences of PAHs on human health. Current epidemiological reports suggest LMW-PAHs may contribute to obesity incidences in children, yet most studies focus on high-molecular-weight PAHs. This study explores 2-naphthol’s impact on obesity and obesity-associated metabolic disorders. To investigate 2-naphthol’s effect on lipid metabolism and inflammation, we employed 3T3-L1 and BAT1 cell lines to model white and brown adipocytes, respectively, alongside a murine macrophage cell line (RAW264.7). We found that 2-naphthol increased the expression of key adipogenic and lipogenic genes while decreasing lipolytic gene expression in chronically treated 3T3-L1 and BAT1 adipocytes. In addition, chronic 2-naphthol treatment also suppressed adrenergic-stimulated thermogenic gene expression in BAT1 brown adipocytes. In consistence, an increase in lipid accumulation was demonstrated in BODIPY and Oil Red O-stained adipocytes. Additionally, 3T3-L1 adipocytes and RAW264.7 macrophages chronically exposed to 2-naphthol showed upregulated mRNA expression of major inflammatory cytokines (e.g., tumor necrosis factor α (
Tnfα), interleukin-1β
(Il-1β), and Il-6). In summary, chronic exposure to 2-naphthol stimulates lipid accumulation in adipocytes and inflammation in adipocytes and macrophages. These findings support previous research that demonstrates 2-naphthol has obesogenic potential.
Full article
►▼
Show Figures
Open AccessArticle
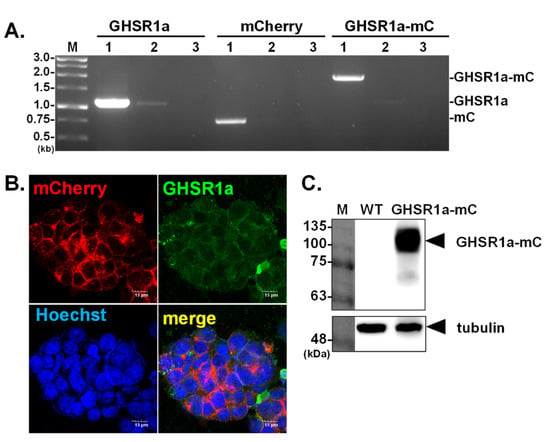
Establishment of a Cell Line Stably Expressing the Growth Hormone Secretagogue Receptor to Identify Crocin as a Ghrelin Agonist
by
Chia-Hao Wang, Ching-Yu Tseng, Wei-Li Hsu and Jason T. C. Tzen
Viewed by 3645
Abstract
The growth hormone secretagogue receptor-1a (GHSR1a) is the endogenous receptor for ghrelin. Activation of GHSR1a participates in many physiological processes including energy homeostasis and eating behavior. Due to its transitory half-life, the efficacy of ghrelin treatment in patients is restricted; hence the development
[...] Read more.
The growth hormone secretagogue receptor-1a (GHSR1a) is the endogenous receptor for ghrelin. Activation of GHSR1a participates in many physiological processes including energy homeostasis and eating behavior. Due to its transitory half-life, the efficacy of ghrelin treatment in patients is restricted; hence the development of new adjuvant therapy is an urgent need. This study aimed to establish a cell line stably expressing GHSR1a, which could be employed to screen potential ghrelin agonists from natural compounds. First, by means of lentiviral transduction, the genome of a human HEK293T cell was modified, and a cell platform stably overexpressing GHSR1a was successfully established. In this platform, GHSR1a was expressed as a fusion protein tagged with mCherry, which allowed the monitoring of the dynamic cellular distribution of GHSR1a by fluorescent microscopy. Subsequently, the authenticity of the GHSR1a mediated signaling was further characterized by using ghrelin and teaghrelin, two molecules known to stimulate GHSR1a. The results indicated that both ghrelin and teaghrelin readily activated GHSR1a mediated signaling pathways, presumably via increasing phosphorylation levels of ERK. The specific GHSR1a signaling was further validated by using SP-analog, an antagonist of GHSR1a as well as using a cell model with the knockdown expression of GHSR1a. Molecular modeling predicted that crocin might be a potential ghrelin agonist, and this prediction was further confirmed by the established platform.
Full article
►▼
Show Figures
Open AccessReview
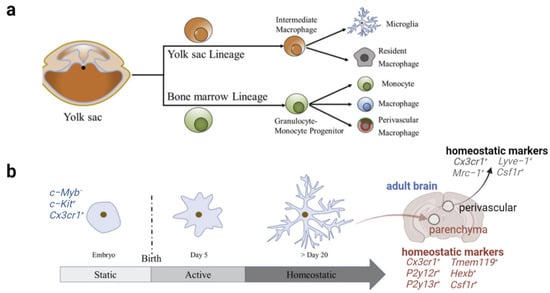
New Insights into Microglial Mechanisms of Memory Impairment in Alzheimer’s Disease
by
Na Li, Mingru Deng, Gonghui Hu, Nan Li, Haicheng Yuan and Yu Zhou
Cited by 15 | Viewed by 3578
Abstract
Alzheimer’s disease (AD) is the most common progressive and irreversible neurodegeneration characterized by the impairment of memory and cognition. Despite years of studies, no effective treatment and prevention strategies are available yet. Identifying new AD therapeutic targets is crucial for better elucidating the
[...] Read more.
Alzheimer’s disease (AD) is the most common progressive and irreversible neurodegeneration characterized by the impairment of memory and cognition. Despite years of studies, no effective treatment and prevention strategies are available yet. Identifying new AD therapeutic targets is crucial for better elucidating the pathogenesis and establishing a valid treatment of AD. Growing evidence suggests that microglia play a critical role in AD. Microglia are resident macrophages in the central nervous system (CNS), and their core properties supporting main biological functions include surveillance, phagocytosis, and the release of soluble factors. Activated microglia not only directly mediate the central immune response, but also participate in the pathological changes of AD, including amyloid-beta (Aβ) aggregation, tau protein phosphorylation, synaptic dissection, neuron loss, memory function decline, etc. Based on these recent findings, we provide a new framework to summarize the role of microglia in AD memory impairment. This evidence suggests that microglia have the potential to become new targets for AD therapy.
Full article
►▼
Show Figures
Open AccessArticle
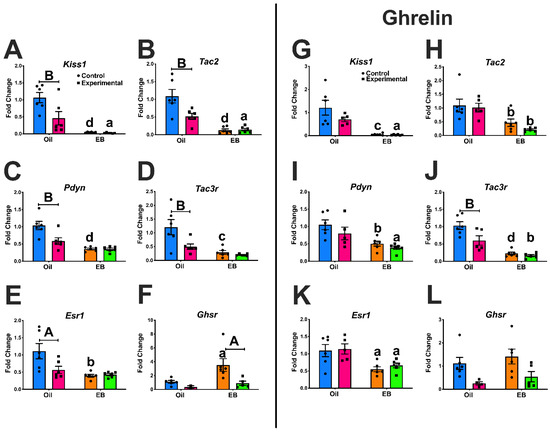
Deletion of Growth Hormone Secretagogue Receptor in Kisspeptin Neurons in Female Mice Blocks Diet-Induced Obesity
by
Kristie Conde, Danielle Kulyk, Allison Vanschaik, Sierra Daisey, Catherine Rojas, Kimberly Wiersielis, Ali Yasrebi, Thomas J. Degroat, Yuxiang Sun and Troy A. Roepke
Cited by 4 | Viewed by 2869
Abstract
The gut peptide, ghrelin, mediates energy homeostasis and reproduction by acting through its receptor, growth hormone secretagogue receptor (GHSR), expressed in hypothalamic neurons in the arcuate (ARC). We have shown 17β-estradiol (E2) increases
Ghsr expression in Kisspeptin/Neurokinin B/Dynorphin (KNDy) neurons, enhancing sensitivity to
[...] Read more.
The gut peptide, ghrelin, mediates energy homeostasis and reproduction by acting through its receptor, growth hormone secretagogue receptor (GHSR), expressed in hypothalamic neurons in the arcuate (ARC). We have shown 17β-estradiol (E2) increases
Ghsr expression in Kisspeptin/Neurokinin B/Dynorphin (KNDy) neurons, enhancing sensitivity to ghrelin. We hypothesized that E2-induced
Ghsr expression augments KNDy sensitivity in a fasting state by elevating ghrelin to disrupt energy expenditure in females. We produced a Kiss1-GHSR knockout to determine the role of GHSR in ARC KNDy neurons. We found that changes in ARC gene expression with estradiol benzoate (EB) treatment were abrogated by the deletion of GHSR and ghrelin abolished these differences. We also observed changes in metabolism and fasting glucose levels. Additionally, knockouts were resistant to body weight gain on a high fat diet (HFD). Behaviorally, we found that knockouts on HFD exhibited reduced anxiety-like behavior. Furthermore, knockouts did not refeed to the same extent as controls after a 24 h fast. Finally, in response to cold stress, knockout females had elevated metabolic parameters compared to controls. These data indicate GHSR in Kiss1 neurons modulate ARC gene expression, metabolism, glucose homeostasis, behavior, and thermoregulation, illustrating a novel mechanism for E2 and ghrelin to control Kiss1 neurons.
Full article
►▼
Show Figures
Open AccessReview
Diverse and Complementary Effects of Ghrelin and Obestatin
by
Daniel Villarreal, Geetali Pradhan, Yu Zhou, Bingzhong Xue and Yuxiang Sun
Cited by 14 | Viewed by 4835
Abstract
Ghrelin and obestatin are two “sibling proteins” encoded by the same preproghrelin gene but possess an array of diverse and complex functions. While there are ample literature documenting ghrelin’s functions, the roles of obestatin are less clear and controversial. Ghrelin and obestatin have
[...] Read more.
Ghrelin and obestatin are two “sibling proteins” encoded by the same preproghrelin gene but possess an array of diverse and complex functions. While there are ample literature documenting ghrelin’s functions, the roles of obestatin are less clear and controversial. Ghrelin and obestatin have been perceived to be antagonistic initially; however, recent studies challenge this dogma. While they have opposing effects in some systems, they function synergistically in other systems, with many functions remaining debatable. In this review, we discuss their functional relationship under three “C” categories, namely complex, complementary, and contradictory. Their functions in food intake, weight regulation, hydration, gastrointestinal motility, inflammation, and insulin secretion are complex. Their functions in pancreatic beta cells, cardiovascular, muscle, neuroprotection, cancer, and digestive system are complementary. Their functions in white adipose tissue, thermogenesis, and sleep regulation are contradictory. Overall, this review accumulates the multifaceted functions of ghrelin and obestatin under both physiological and pathological conditions, with the intent of contributing to a better understanding of these two important gut hormones.
Full article
►▼
Show Figures
Open AccessReview
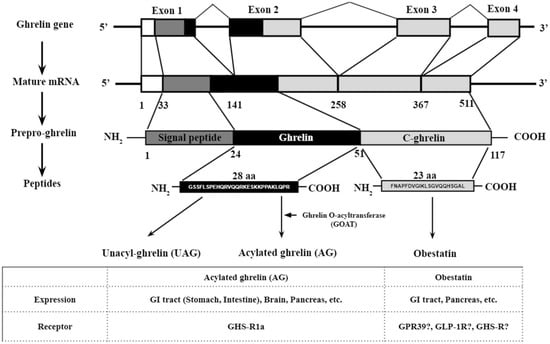
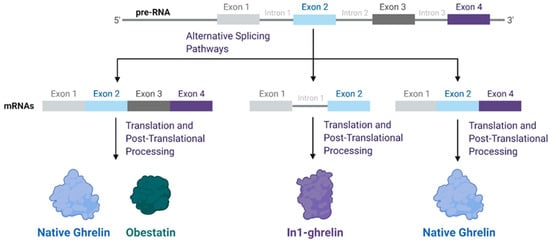
Ghrelin and Cancer: Examining the Roles of the Ghrelin Axis in Tumor Growth and Progression
by
Anuhya S. Kotta, Abigail S. Kelling, Karen A. Corleto, Yuxiang Sun and Erin D. Giles
Cited by 12 | Viewed by 4077
Abstract
Ghrelin, a hormone produced and secreted from the stomach, is prim arily known as an appetite stimulant. Recently, it has emerged as a potential regulator/biomarker of cancer progression. Inconsistent results on this subject make this body of literature difficult to interpret. Here, we
[...] Read more.
Ghrelin, a hormone produced and secreted from the stomach, is prim arily known as an appetite stimulant. Recently, it has emerged as a potential regulator/biomarker of cancer progression. Inconsistent results on this subject make this body of literature difficult to interpret. Here, we attempt to identify commonalities in the relationships between ghrelin and various cancers, and summarize important considerations for future research. The main players in the ghrelin family axis are unacylated ghrelin (UAG), acylated ghrelin (AG), the enzyme ghrelin O-acyltransferase (GOAT), and the growth hormone secretagogue receptor (GHSR). GOAT is responsible for the acylation of ghrelin, after which ghrelin can bind to the functional ghrelin receptor GHSR-1a to initiate the activation cascade. Splice variants of ghrelin also exist, with the most prominent being In1-ghrelin. In this review, we focus primarily on the potential of In1-ghrelin as a biomarker for cancer progression, the unique characteristics of UAG and AG, the importance of the two known receptor variants GHSR-1a and 1b, as well as the possible mechanisms through which the ghrelin axis acts. Further understanding of the role of the ghrelin axis in tumor cell proliferation could lead to the development of novel therapeutic approaches for various cancers.
Full article
►▼
Show Figures
Open AccessArticle
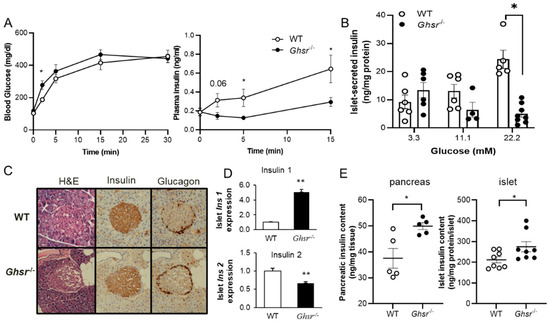
Mechanistic Investigation of GHS-R Mediated Glucose-Stimulated Insulin Secretion in Pancreatic Islets
by
Geetali Pradhan, Jong Han Lee, Chia-Shan Wu, Hongying Wang, Ligen Lin, Taraka Donti, Brett H. Graham, Arun S. Rajan, Ashok Balasubramanyam, Susan L. Samson, Shaodong Guo and Yuxiang Sun
Cited by 5 | Viewed by 3899
Abstract
Ghrelin receptor, a growth hormone secretagogue receptor (GHS-R), is expressed in the pancreas. Emerging evidence indicates that GHS-R is involved in the regulation of glucose-stimulated insulin secretion (GSIS), but the mechanism by which GHS-R regulates GSIS in the pancreas is unclear. In this
[...] Read more.
Ghrelin receptor, a growth hormone secretagogue receptor (GHS-R), is expressed in the pancreas. Emerging evidence indicates that GHS-R is involved in the regulation of glucose-stimulated insulin secretion (GSIS), but the mechanism by which GHS-R regulates GSIS in the pancreas is unclear. In this study, we investigated the role of GHS-R on GSIS in detail using global
Ghsr−/− mice (in vivo) and
Ghsr-ablated pancreatic islets (ex vivo). GSIS was attenuated in both
Ghsr−/− mice and
Ghsr-ablated islets, while the islet morphology was similar between WT and
Ghsr−/− mice. To elucidate the mechanism underpinning
Ghsr-mediated GSIS, we investigated the key steps of the GSIS signaling cascade. The gene expression of glucose transporter 2 (
Glut2) and the glucose-metabolic intermediate—glucose-6-phosphate (G6P) were reduced in
Ghsr-ablated islets, supporting decreased glucose uptake. There was no difference in mitochondrial DNA content in the islets of WT and
Ghsr−/− mice, but the ATP/ADP ratio in
Ghsr−/− islets was significantly lower than that of WT islets. Moreover, the expression of pancreatic and duodenal homeobox 1 (Pdx1), as well as insulin signaling genes of insulin receptor (IR) and insulin receptor substrates 1 and 2 (IRS1/IRS2), was downregulated in
Ghsr−/− islets. Akt is the key mediator of the insulin signaling cascade. Concurrently, Akt phosphorylation was reduced in the pancreas of
Ghsr−/− mice under both insulin-stimulated and homeostatic conditions. These findings demonstrate that GHS-R ablation affects key components of the insulin signaling pathway in the pancreas, suggesting the existence of a cross-talk between GHS-R and the insulin signaling pathway in pancreatic islets, and GHS-R likely regulates GSIS via the Akt-Pdx1-GLUT2 pathway.
Full article
►▼
Show Figures
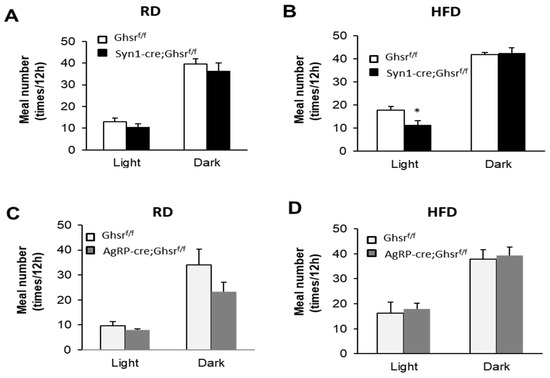
Open AccessCommunication
Neuronal GHS-R Differentially Modulates Feeding Patterns under Normal and Obesogenic Conditions
by
Jong Han Lee, Bingzhong Xue, Zheng Chen and Yuxiang Sun
Cited by 2 | Viewed by 2540
Abstract
The orexigenic hormone ghrelin increases food intake and promotes obesity through its receptor, growth hormone secretagogue receptor (GHS-R). We previously reported two neuron-specific GHS-R knockout mouse lines, namely pan-neuronal deletion by Syn1-cre and hypothalamic deletion by AgRP-cre, exhibiting differential diet-dependent effects on body
[...] Read more.
The orexigenic hormone ghrelin increases food intake and promotes obesity through its receptor, growth hormone secretagogue receptor (GHS-R). We previously reported two neuron-specific GHS-R knockout mouse lines, namely pan-neuronal deletion by Syn1-cre and hypothalamic deletion by AgRP-cre, exhibiting differential diet-dependent effects on body weight. GHS-R deficiency in neurons elicited less pronounced metabolic effects under regular diet (RD) than high fat diet (HFD). While there was no difference in total food intake of HFD in either mouse line, Syn1-cre; Ghsr
f/f mice showed much greater anti-obesity effect than that of AgRP-cre; Ghsr
f/f mice. Meal feeding pattern is known to have a major impact on energy homeostasis and obesity development. Here, we investigated the feeding behaviors of these two neuron-specific GHS-R knockout mice under RD and HFD feeding, by assessing meal number, meal size, meal duration, and feeding frequency. Under the normal diet, RD-fed Syn1-cre; Ghsr
f/f mice showed a decreased meal size in dark phase, while RD-fed AgRP-cre; Ghsr
f/f mice showed an increased meal duration in dark phase. Under the obesogenic diet, HFD-fed Syn1-cre; Ghsr
f/f mice displayed reduced meal numbers in light phase and increased feeding in both light and dark phases, whereas HFD-fed AgRP-cre; Ghsr
f/f mice showed a decreased meal duration in the light phase only. Consistently, the expression of neuropeptides (Neuropeptide Y and Orexin) was increased in the hypothalamus of RD-fed Syn1-cre; Ghsr
f/f mice, whereas the expression of cannabinoid receptor type 1 (CB1) was increased in the hypothalamus of HFD fed Syn1-cre; Ghsr
f/f mice. Overall, feeding pattern changes were more pronounced in Syn1-cre; Ghsr
f/f mice than that in AgRP-cre; Ghsr
f/f mice, and HFD elicited greater alteration than RD. While AgRP-cre; Ghsr
f/f mice consumed HFD meals faster during the day (showing shorter meal duration), Syn1-cre; Ghsr
f/f mice ate few HFD meals during the light phase and ate slowly throughout the day (showing longer meal duration in both phases). Our findings reveal that neuronal GHS-R regulates energy homeostasis by altering feeding patterns, and differentially modulates feeding patterns in a site- and diet-dependent manner. The distinctive data in these two mouse lines also suggest that eating slowly during the optimal feeding period (dark phase for mice) may be beneficial in combating obesity.
Full article
►▼
Show Figures
Open AccessArticle
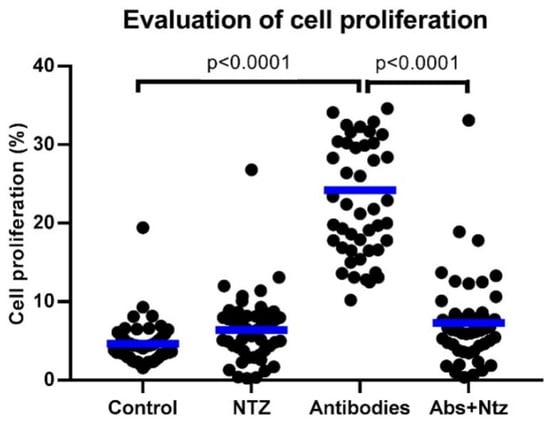
Nitazoxanide Exerts Immunomodulatory Effects on Peripheral Blood Mononuclear Cells from Type 2 Diabetes Patients
by
Mauricio Castillo-Salazar, Fausto Sánchez-Muñoz, Rashidi Springall del Villar, Gabriel Navarrete-Vázquez, Adrián Hernández-DiazCouder, Carlos Mojica-Cardoso, Sara García-Jiménez, Cairo Toledano-Jaimes and Germán Bernal-Fernández
Cited by 8 | Viewed by 3046
Abstract
Background: Type 2 diabetes (T2D) is a low-grade inflammatory condition with abnormalities in the immune response mediated by T lymphocytes and macrophages. Drug repositioning for immunomodulatory molecules is an attractive proposal for treating T2D. Nitazoxanide (NTZ) is a broad-spectrum drug with promising immunomodulatory
[...] Read more.
Background: Type 2 diabetes (T2D) is a low-grade inflammatory condition with abnormalities in the immune response mediated by T lymphocytes and macrophages. Drug repositioning for immunomodulatory molecules is an attractive proposal for treating T2D. Nitazoxanide (NTZ) is a broad-spectrum drug with promising immunomodulatory effects. Thus, we investigated the immunomodulatory effect of NTZ on peripheral blood mononuclear cells (PBMCs) from patients with T2D. Methods: Fifty patients with T2D were selected, and the proliferative response of T lymphocytes and the M1/M2 ratio of macrophages post cell culture were evaluated by flow cytometry, as well as measuring the concentration of cytokines by ELISA and the relative expression of microRNAs (miRNAs) related to the immune response by real-time PCR. Results: NTZ exerts an inhibitory effect on the cell proliferation of T lymphocytes stimulated with anti-CD3 and anti-CD28 antibodies without modifying cell viability, and significant decreases in the supernatant concentrations of interleukin (IL)-1β, IL-2, IL-6, IL-10, and IL-12. Furthermore, NTZ negatively regulates the relative expression of miR-155-5p without changes in miR-146a-5p. The M1/M2 ratio of monocytes/macrophages decreased the M1 and increased the M2 subpopulation by NTZ. Conclusions: Our results suggest that NTZ exerts immunomodulatory effects on PBMCs from T2D patients, and shows potential alternative therapeutic benefits.
Full article
►▼
Show Figures
Open AccessArticle
High Glucose Concentrations Impair the Processing and Presentation of Mycobacterium tuberculosis Antigens In Vitro
by
Guadalupe Monroy-Mérida, Silvia Guzmán-Beltrán, Fernando Hernández, Teresa Santos-Mendoza and Karen Bobadilla
Cited by 7 | Viewed by 2615
Abstract
Type 2 diabetes is an established risk factor for tuberculosis, but the underlying mechanisms are largely unknown. We established an in vitro model to analyze the effect of high glucose concentrations in antigen processing and presentation in antigen-presenting cells. Human monocyte-derived macrophages (MDMs)
[...] Read more.
Type 2 diabetes is an established risk factor for tuberculosis, but the underlying mechanisms are largely unknown. We established an in vitro model to analyze the effect of high glucose concentrations in antigen processing and presentation in antigen-presenting cells. Human monocyte-derived macrophages (MDMs) were exposed to high (11 mM and 30 mM) and low (5.5 mM) glucose concentrations and infected with
Mycobacterium tuberculosis (Mtb). Flow cytometry was used to analyze the effect of high glucose concentrations in histocompatibility complex (MHC) class II molecules (HLA-DR) and co-stimulatory molecules (CD80 and CD86), indispensable for an adequate antigenic presentation and CD4+ T cell activation. HLA-DR and CD86 were significantly decreased by high glucose concentrations compared with low glucose concentrations. Confocal microscopy was used to detect Rab 5 and Lamp-1, proteins involved in the kinetics of antigen processing as early markers, and Rab 7 and cathepsin D as late markers. We observed a delay in the dynamics of the acquisition of Rab 7 and cathepsin D in high glucose concentrations. Moreover, the kinetics of the formation
M. tuberculosis peptide–MHC II complexes in MDMs was decreased under high glucose concentrations, reducing their capacity for T cell activation. These findings suggest that high glucose concentrations directly affect antigenic processing, and therefore antigenic presentation.
Full article
►▼
Show Figures
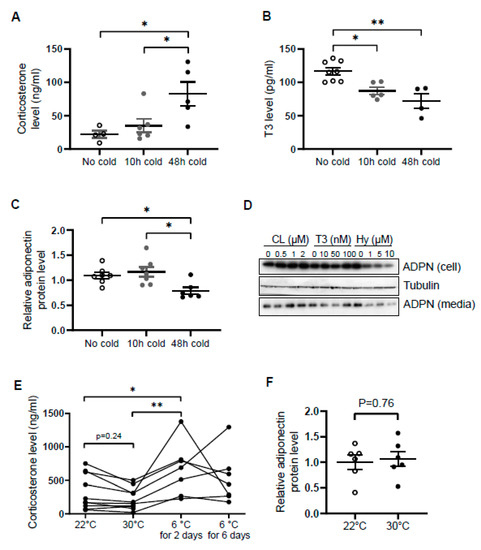
Open AccessArticle
Glucocorticoid/Adiponectin Axis Mediates Full Activation of Cold-Induced Beige Fat Thermogenesis
by
Liping Luo, Lu Wang, Yan Luo, Estevan Romero, Xin Yang and Meilian Liu
Cited by 3 | Viewed by 3333
Abstract
Glucocorticoids (GCs), a class of corticosteroids produced by the adrenal cortex in response to stress, exert obesity-promoting effects. Although adaptive thermogenesis has been considered an effective approach to counteract obesity, whether GCs play a role in regulating cold stress-induced thermogenesis remains incompletely understood.
[...] Read more.
Glucocorticoids (GCs), a class of corticosteroids produced by the adrenal cortex in response to stress, exert obesity-promoting effects. Although adaptive thermogenesis has been considered an effective approach to counteract obesity, whether GCs play a role in regulating cold stress-induced thermogenesis remains incompletely understood. Here, we show that the circulating levels of stress hormone corticosterone (GC in rodents) were significantly elevated, whereas the levels of adiponectin, an adipokine that was linked to cold-induced adaptive thermogenesis, were decreased 48 h post cold exposure. The administration of a glucocorticoid hydrocortisone downregulated adiponectin protein and mRNA levels in both WAT and white adipocytes, and upregulated thermogenic gene expression in inguinal fat. In contrast, mifepristone, a glucocorticoid receptor antagonist, enhanced adiponectin expression and suppressed energy expenditure in vivo. Mechanistically, hydrocortisone suppressed adiponectin expression by antagonizing PPARγ in differentiated 3T3-L1 adipocytes. Ultimately, adiponectin deficiency restored mifepristone-decreased oxygen consumption and suppressed the expression of thermogenic genes in inguinal fat. Taken together, our study reveals that the GCs/adiponectin axis is a key regulator of beige fat thermogenesis in response to acute cold stress.
Full article
►▼
Show Figures
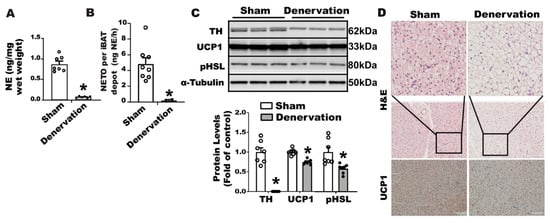
Open AccessEditor’s ChoiceArticle
Fatty Acids Rescue the Thermogenic Function of Sympathetically Denervated Brown Fat
by
Qiang Cao, Shirong Wang, Huan Wang, Xin Cui, Jia Jing, Liqing Yu, Hang Shi and Bingzhong Xue
Cited by 5 | Viewed by 2959
Abstract
Sympathetic nervous system (SNS) innervation into brown adipose tissue (BAT) has been viewed as an impetus for brown fat thermogenesis. However, we surprisingly discovered that BAT SNS innervation is dispensable for mice to maintain proper body temperature during a prolonged cold exposure. Here
[...] Read more.
Sympathetic nervous system (SNS) innervation into brown adipose tissue (BAT) has been viewed as an impetus for brown fat thermogenesis. However, we surprisingly discovered that BAT SNS innervation is dispensable for mice to maintain proper body temperature during a prolonged cold exposure. Here we aimed to uncover the physiological factors compensating for maintaining brown fat thermogenesis in the absence of BAT innervation. After an initial decline of body temperature during cold exposure, mice with SNS surgical denervation in interscapular BAT gradually recovered their temperature comparable to that of sham-operated mice. The surgically denervated BAT also maintained a sizable uncoupling protein 1 (UCP1) protein along with basal norepinephrine (NE) at a similar level to that of sham controls, which were associated with increased circulating NE. Furthermore, the denervated mice exhibited increased free fatty acid levels in circulation. Indeed, surgical denervation of mice with CGI-58 deletion in adipocytes, a model lacking lipolytic capacity to release fatty acids from WAT, dramatically reduced BAT UCP1 protein and rendered the mice susceptible to cold. We conclude that circulating fatty acids and NE may serve as key factors for maintaining BAT thermogenic function and body temperature in the absence of BAT sympathetic innervation.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceReview
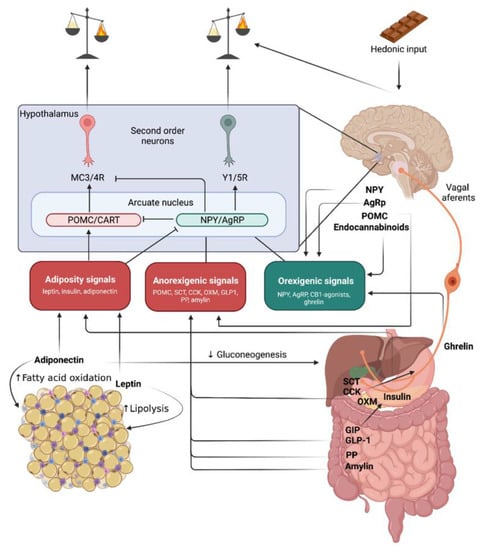
Obesity–An Update on the Basic Pathophysiology and Review of Recent Therapeutic Advances
by
Erind Gjermeni, Anna S. Kirstein, Florentien Kolbig, Michael Kirchhof, Linnaeus Bundalian, Julius L. Katzmann, Ulrich Laufs, Matthias Blüher, Antje Garten and Diana Le Duc
Cited by 46 | Viewed by 24065
Abstract
Obesity represents a major public health problem with a prevalence increasing at an alarming rate worldwide. Continuous intensive efforts to elucidate the complex pathophysiology and improve clinical management have led to a better understanding of biomolecules like gut hormones, antagonists of orexigenic signals,
[...] Read more.
Obesity represents a major public health problem with a prevalence increasing at an alarming rate worldwide. Continuous intensive efforts to elucidate the complex pathophysiology and improve clinical management have led to a better understanding of biomolecules like gut hormones, antagonists of orexigenic signals, stimulants of fat utilization, and/or inhibitors of fat absorption. In this article, we will review the pathophysiology and pharmacotherapy of obesity including intersection points to the new generation of antidiabetic drugs. We provide insight into the effectiveness of currently approved anti-obesity drugs and other therapeutic avenues that can be explored.
Full article
►▼
Show Figures
Open AccessArticle
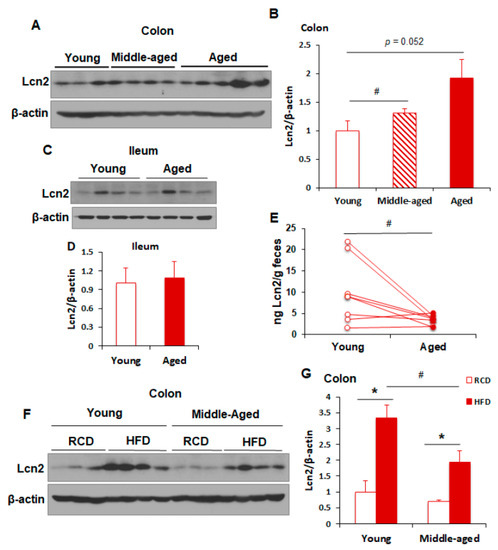
Lipocalin 2 Deficiency Restrains Aging-Related Reshaping of Gut Microbiota Structure and Metabolism
by
Xiaoxue Qiu, Chi Chen and Xiaoli Chen
Cited by 5 | Viewed by 3101
Abstract
Gut microbiota modulate age-associated changes in metabolism, innate immune responses, and cognitive function. However, the involvement of host factors in the regulation of age-dependent gut microbial structure and intestinal inflammation is largely unknown. Lipocalin 2 (Lcn2) has previously been identified as an adipocytokine
[...] Read more.
Gut microbiota modulate age-associated changes in metabolism, innate immune responses, and cognitive function. However, the involvement of host factors in the regulation of age-dependent gut microbial structure and intestinal inflammation is largely unknown. Lipocalin 2 (Lcn2) has previously been identified as an adipocytokine and characterized as an important regulator of diet-induced obesity and inflammation. Previous studies have shown that Lcn2 plays a role in high fat diet-induced reshaping of gut microbiota and intestinal inflammation. However, the role of Lcn2 in the regulation of aging-related reshaping of gut microbiota is unclear. Herein, we demonstrate that fecal levels of Lcn2 are reduced during aging. Age reshaped gut microbiota composition in wild-type (WT) mice. Interestingly, Lcn2 deficiency diminished this effect of aging in Lcn2 knockout (LKO) mice, leading to decreased bacterial diversity and increased Firmicutes to Bacteroidetes (F to B) ratio. Specifically, we identified 16 bacteria at the family level that were differentially abundant between WT and LKO mice at old age. Several health-promoting bacteria, including SCFA-producing bacteria, were significantly less prevalent in old LKO mice compared to WT mice, indicating that Lcn2 deficiency shifts the aging-related gut microbial community towards an unhealthy population and lowers microbial butyrate production. Our results provide a line of evidence that Lcn2 plays a role in the control of aging-related reshaping of gut microbiota composition and metabolites.
Full article
►▼
Show Figures
Open AccessArticle
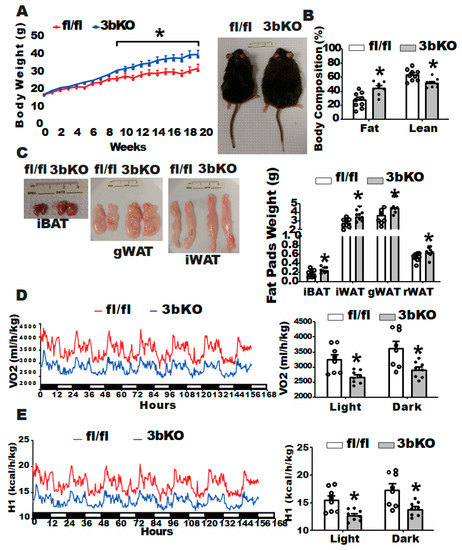
Dnmt3b Deficiency in Myf5+-Brown Fat Precursor Cells Promotes Obesity in Female Mice
by
Shirong Wang, Qiang Cao, Xin Cui, Jia Jing, Fenfen Li, Huidong Shi, Bingzhong Xue and Hang Shi
Cited by 8 | Viewed by 3632
Abstract
Increasing energy expenditure through activation of brown fat thermogenesis is a promising therapeutic strategy for the treatment of obesity. Epigenetic regulation has emerged as a key player in regulating brown fat development and thermogenic program. Here, we aimed to study the role of
[...] Read more.
Increasing energy expenditure through activation of brown fat thermogenesis is a promising therapeutic strategy for the treatment of obesity. Epigenetic regulation has emerged as a key player in regulating brown fat development and thermogenic program. Here, we aimed to study the role of DNA methyltransferase 3b (Dnmt3b), a DNA methyltransferase involved in de novo DNA methylation, in the regulation of brown fat function and energy homeostasis. We generated a genetic model with
Dnmt3b deletion in brown fat-skeletal lineage precursor cells (3bKO mice) by crossing
Dnmt3b-floxed (fl/fl) mice with
Myf5-Cre mice. Female 3bKO mice are prone to diet-induced obesity, which is associated with decreased energy expenditure. Dnmt3b deficiency also impairs cold-induced thermogenic program in brown fat. Surprisingly, further RNA-seq analysis reveals a profound up-regulation of myogenic markers in brown fat of 3bKO mice, suggesting a myocyte-like remodeling in brown fat. Further motif enrichment and pyrosequencing analysis suggests myocyte enhancer factor 2C (
Mef2c) as a mediator for the myogenic alteration in
Dnmt3b-deficient brown fat, as indicated by decreased methylation at its promoter. Our data demonstrate that brown fat
Dnmt3b is a key regulator of brown fat development, energy metabolism and obesity in female mice.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceArticle
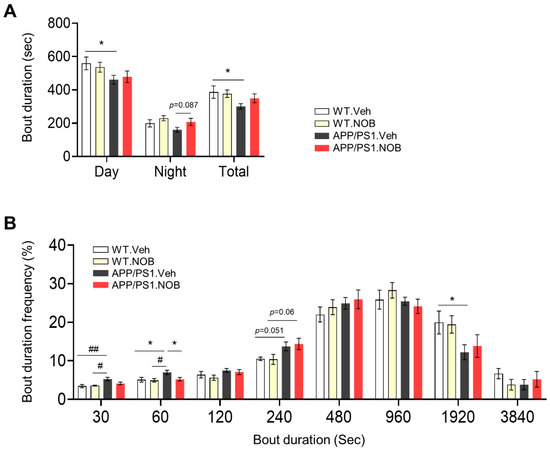
Effects of the Clock Modulator Nobiletin on Circadian Rhythms and Pathophysiology in Female Mice of an Alzheimer’s Disease Model
by
Eunju Kim, Kazunari Nohara, Marvin Wirianto, Gabriel Escobedo, Jr., Ji Ye Lim, Rodrigo Morales, Seung-Hee Yoo and Zheng Chen
Cited by 33 | Viewed by 4983
Abstract
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder and the most common cause of dementia. Various pathogenic mechanisms have been proposed to contribute to disease progression, and recent research provided evidence linking dysregulated circadian rhythms/sleep and energy metabolism with AD. Previously, we found
[...] Read more.
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder and the most common cause of dementia. Various pathogenic mechanisms have been proposed to contribute to disease progression, and recent research provided evidence linking dysregulated circadian rhythms/sleep and energy metabolism with AD. Previously, we found that the natural compound Nobiletin (NOB) can directly activate circadian cellular oscillators to promote metabolic health in disease models and healthy aging in naturally aged mice. In the current study, using the amyloid-β AD model APP/PS1, we investigated circadian, metabolic and amyloid characteristics of female mice and the effects of NOB. Female APP/PS1 mice showed reduced sleep bout duration, and NOB treatment exhibited a trend to improve it. While glucose tolerance was unchanged, female APP/PS1 mice displayed exaggerated oxygen consumption and CO2 production, which was mitigated by NOB. Likewise, cold tolerance in APP/PS1 was impaired relative to WT, and interestingly was markedly enhanced in NOB-treated APP/PS1 mice. Although circadian behavioral rhythms were largely unchanged, real-time qPCR analysis revealed altered expression of several core clock genes by NOB in the cerebral cortex, notably
Bmal1,
Npas2, and
Rora. Moreover, NOB was also able to activate various clock-controlled metabolic genes involved in insulin signaling and mitochondrial function, including
Igf1,
Glut1,
Insr,
Irs1,
Ucp2, and
Ucp4. Finally, we observed that NOB attenuated the expression of several AD related genes including
App,
Bace1, and
ApoE, reduced APP protein levels, and strongly ameliorated Aβ pathology in the cortex. Collectively, these results reveal novel genotype differences and importantly beneficial effects of a natural clock-enhancing compound in biological rhythms and related pathophysiology, suggesting the circadian clock as a modifiable target for AD.
Full article
►▼
Show Figures
Open AccessArticle
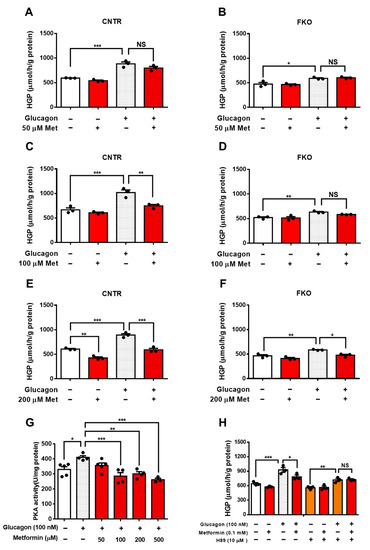
Metformin Targets Foxo1 to Control Glucose Homeostasis
by
Xiaoqin Guo, Xiaopeng Li, Wanbao Yang, Wang Liao, James Zheng Shen, Weiqi Ai, Quan Pan, Yuxiang Sun, Kebin Zhang, Rui Zhang, Yuyang Qiu, Qian Dai, Hongting Zheng and Shaodong Guo
Cited by 9 | Viewed by 3728
Abstract
Metformin is the first-line pharmacotherapy for type 2 diabetes mellitus (T2D). Metformin exerts its glucose-lowering effect primarily through decreasing hepatic glucose production (HGP). However, the precise molecular mechanisms of metformin remain unclear due to supra-pharmacological concentration of metformin used in the study. Here,
[...] Read more.
Metformin is the first-line pharmacotherapy for type 2 diabetes mellitus (T2D). Metformin exerts its glucose-lowering effect primarily through decreasing hepatic glucose production (HGP). However, the precise molecular mechanisms of metformin remain unclear due to supra-pharmacological concentration of metformin used in the study. Here, we investigated the role of Foxo1 in metformin action in control of glucose homeostasis and its mechanism via the transcription factor Foxo1 in mice, as well as the clinical relevance with co-treatment of aspirin. We showed that metformin inhibits HGP and blood glucose in a Foxo1-dependent manner. Furthermore, we identified that metformin suppresses glucagon-induced HGP through inhibiting the PKA→Foxo1 signaling pathway. In both cells and mice, Foxo1-S273D or A mutation abolished the suppressive effect of metformin on glucagon or fasting-induced HGP. We further showed that metformin attenuates PKA activity, decreases Foxo1-S273 phosphorylation, and improves glucose homeostasis in diet-induced obese mice. We also provided evidence that salicylate suppresses HGP and blood glucose through the PKA→Foxo1 signaling pathway, but it has no further additive improvement with metformin in control of glucose homeostasis. Our study demonstrates that metformin inhibits HGP through PKA-regulated transcription factor Foxo1 and its S273 phosphorylation.
Full article
►▼
Show Figures
Planned Papers
The below list represents only planned manuscripts. Some of these
manuscripts have not been received by the Editorial Office yet. Papers
submitted to MDPI journals are subject to peer-review.
Title: Mammalian Suprachiasmatic Nuclei-derived Extracellular Vesicle: Identification and Characterization
Authors: Yuhua Farnell
Affiliation: Department of Poultry Science, Texas A&M AgriLife Research, Texas A&M University, College Station, TX 77843, USA
Title: To be determined
Authors: Qun Sophia Zang; et al.
Affiliation: Department of Surgery, Burn & Shock Trauma Research Institute, Loyola University Chicago Stritch School of Medicine, Maywood, IL, USA
Title: To be determined
Authors: Lily Q Dong; et al.
Affiliation: Department of Cell Systems and Anatomy, University of Texas Health at San Antonio, San Antonio, TX, USA