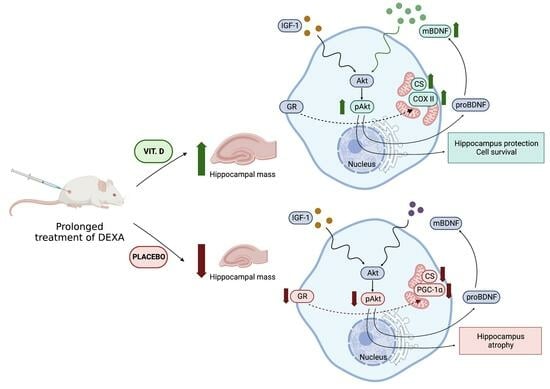
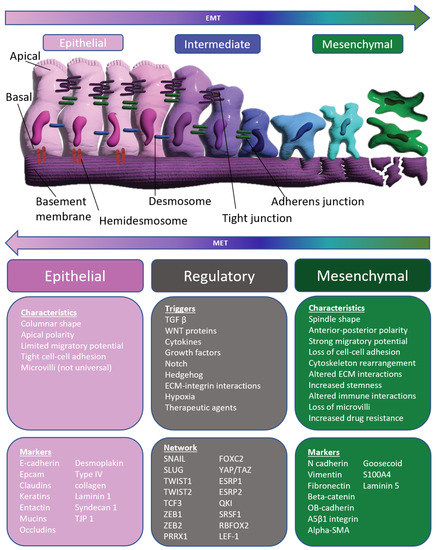
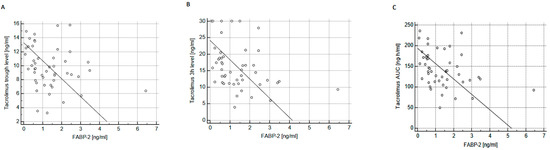
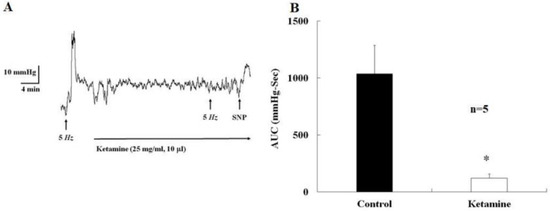
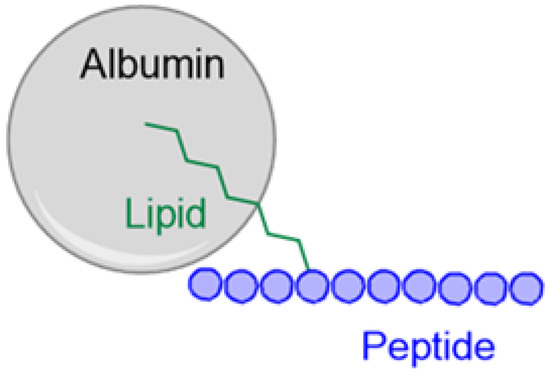
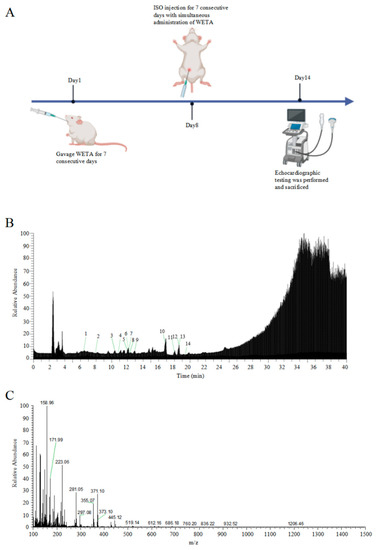
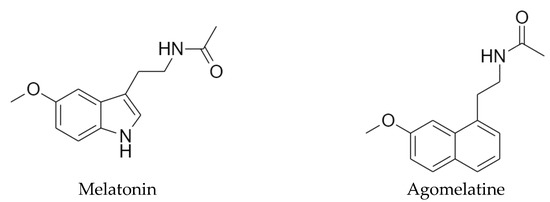
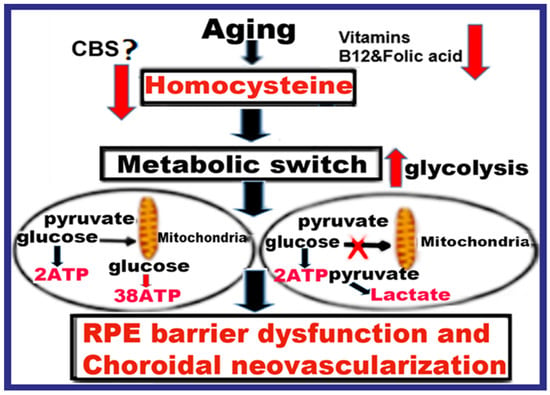
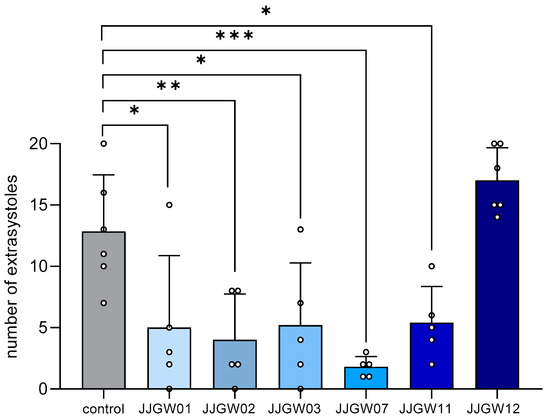
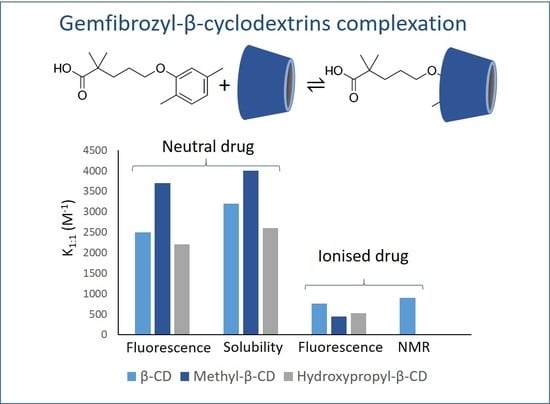
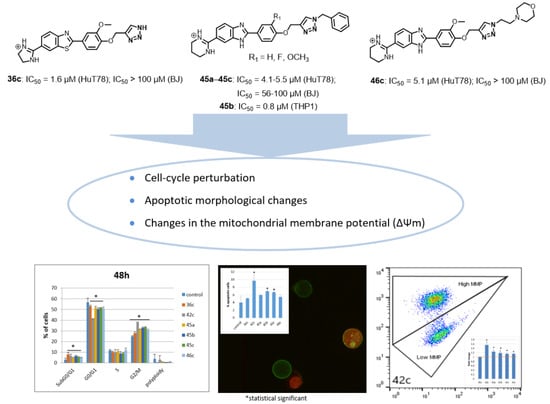
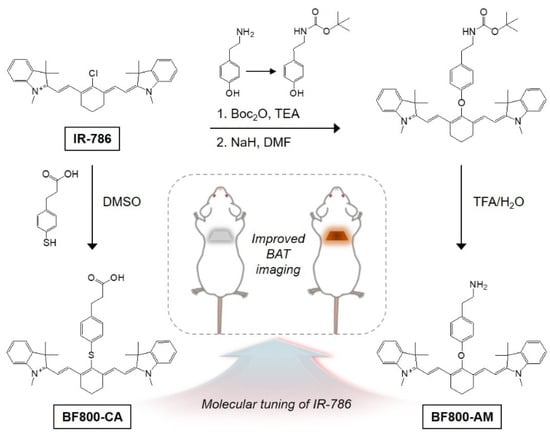
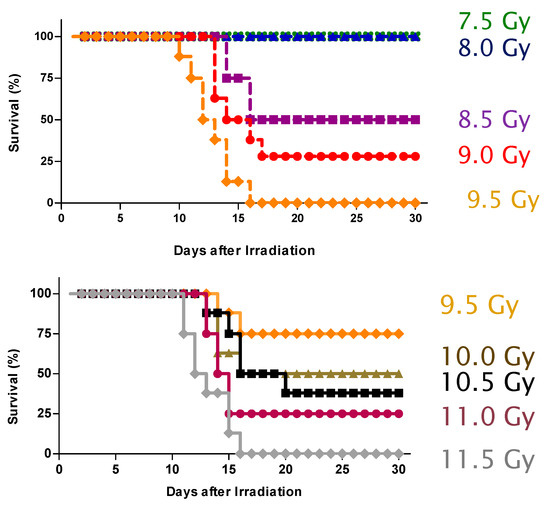
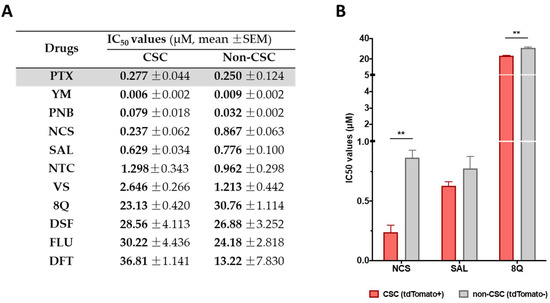
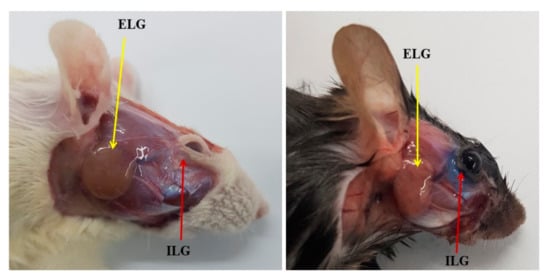
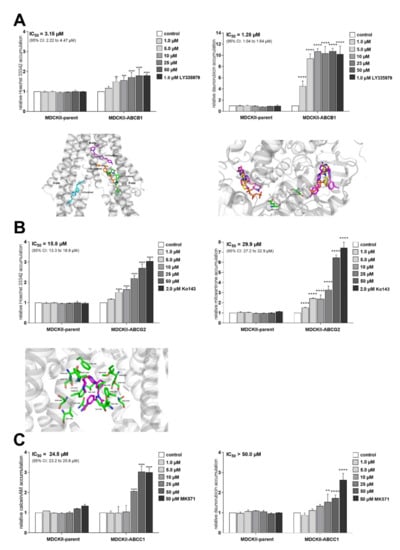
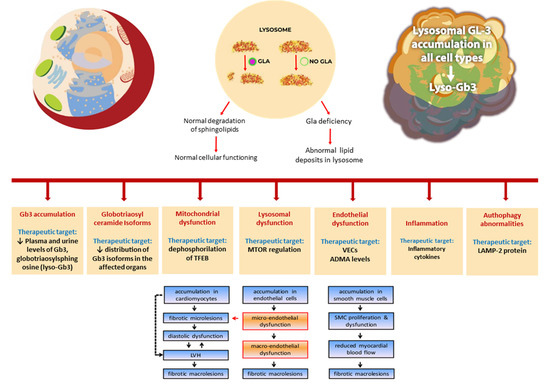
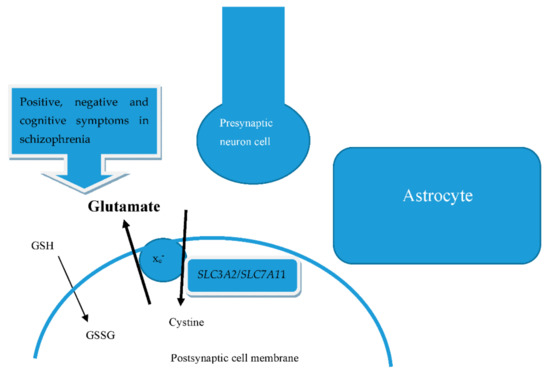
Feature Papers in Molecular Pharmacology
A topical collection in International Journal of Molecular Sciences (ISSN 1422-0067). This collection belongs to the section "Molecular Pharmacology".
Viewed by 294673Editor
Interests: neuropharmacology; neurotoxicology; purinergic mechanisms; modulation of synaptic transmission; glial cells; necrosis/apoptosis; analgesia; learning and memory; epileptic state
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
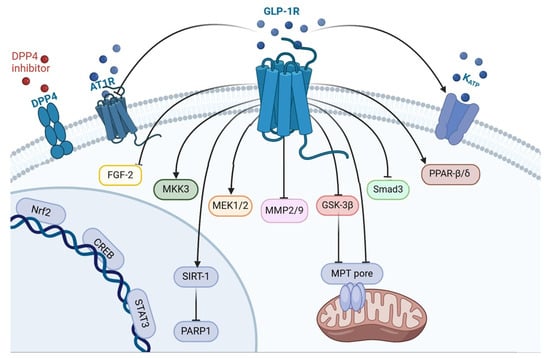
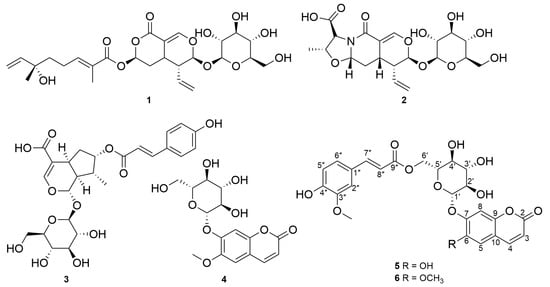
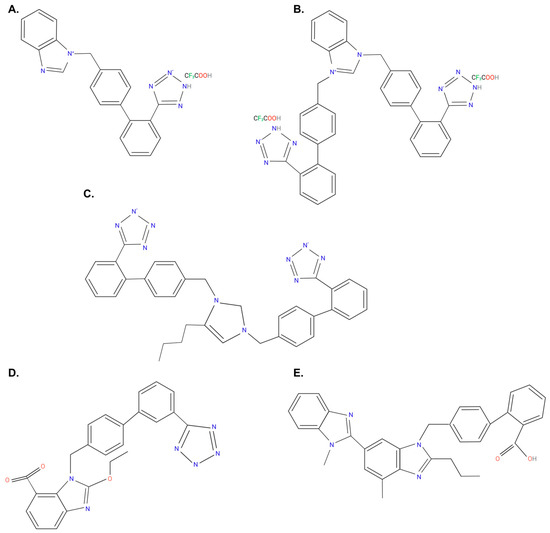
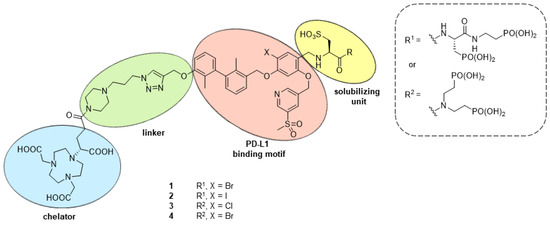
The Topical Collection entitled “Feature Papers in Molecular Pharmacology” aims to collect high-quality research articles and reviews in the field of molecular pharmacology. We encourage Editorial Board Members of this IJMS Section to submit manuscripts reporting on significant advances in molecular pharmacology research. Special emphasis will be placed on drug effects upon nucleic acids, proteins involved in nucleic acid metabolism, and interaction of nucleic acids with proteins, by using the whole plethora of molecular biology methods. Genetic and epigenetic factors determining drug effects will be accordingly dealt with.
Prof. Dr. Peter Illes
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. International Journal of Molecular Sciences is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. There is an Article Processing Charge (APC) for publication in this open access journal. For details about the APC please see here. Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.