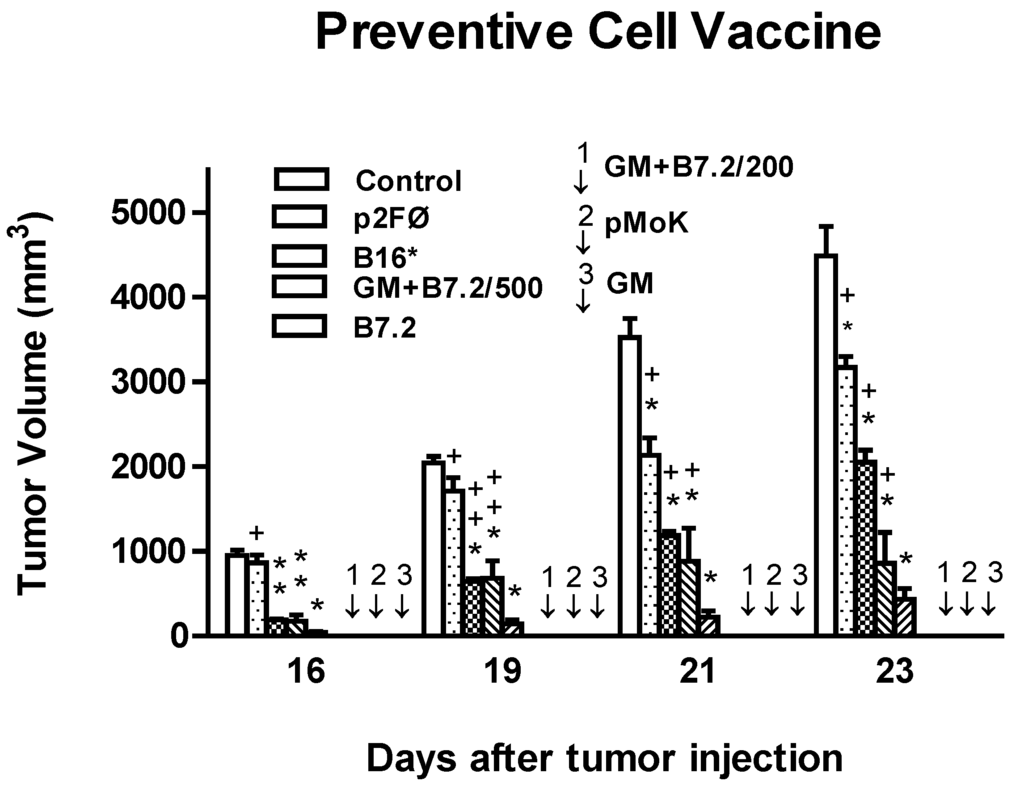
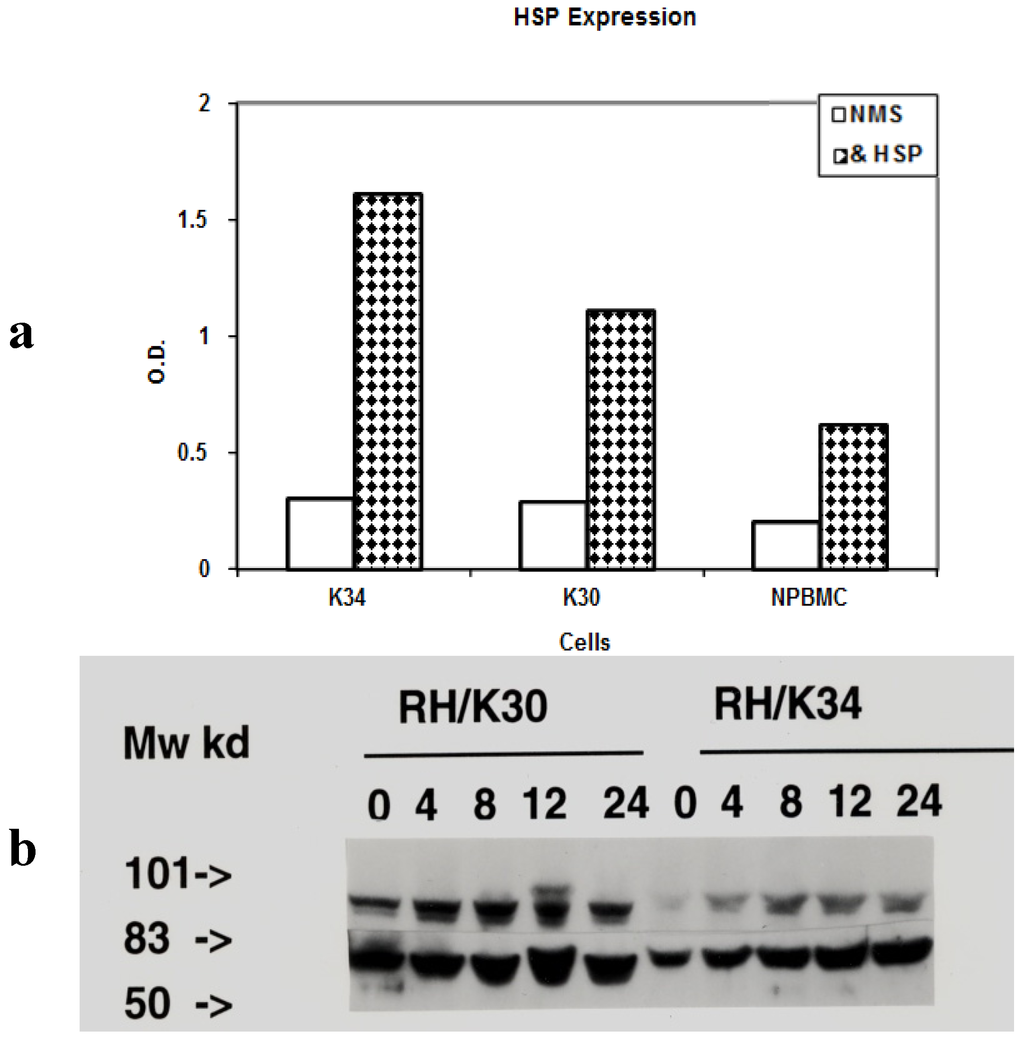
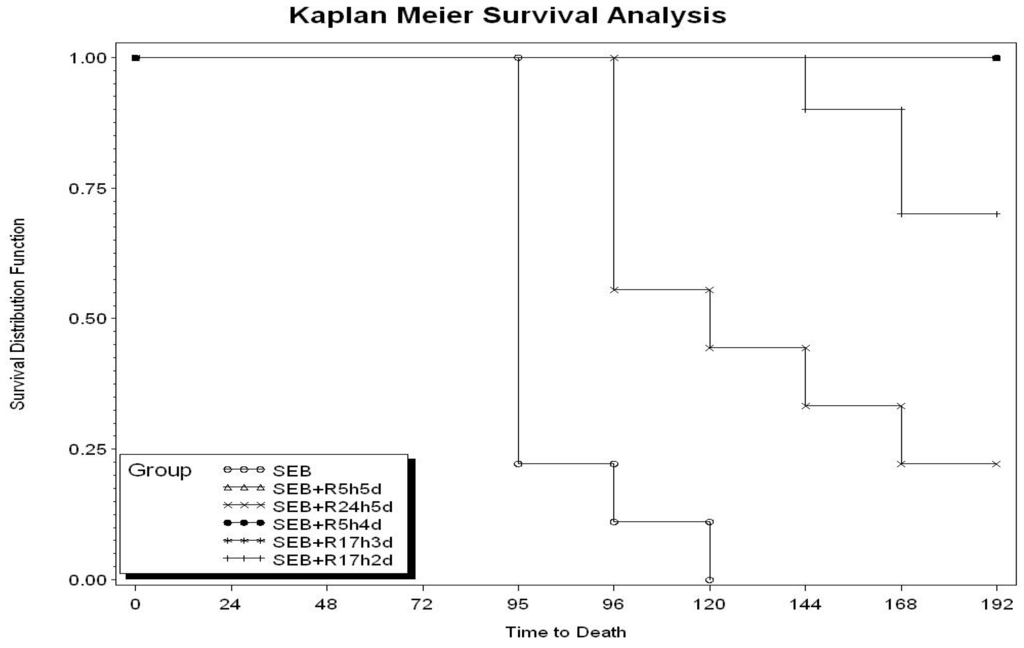
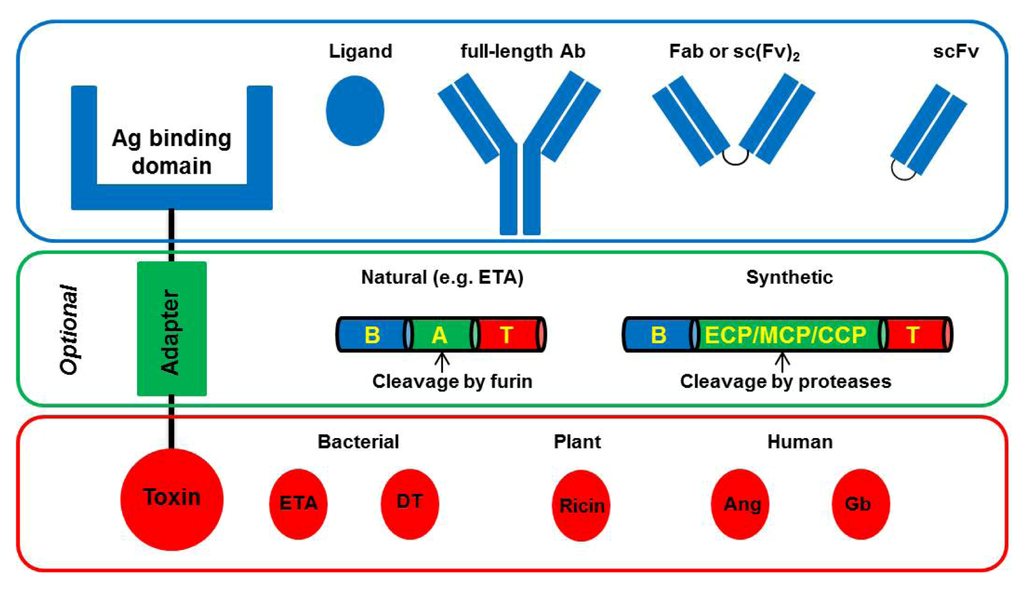
Toxicity and Therapeutic Interventions in the Immune System
A topical collection in Toxins (ISSN 2072-6651).
Viewed by 292233Editor
Interests: cancer; oncology; multiple sclerosis; autoimmune diseases; immunology; chemokines; hematology; drug mechanisms of action (MOA)
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
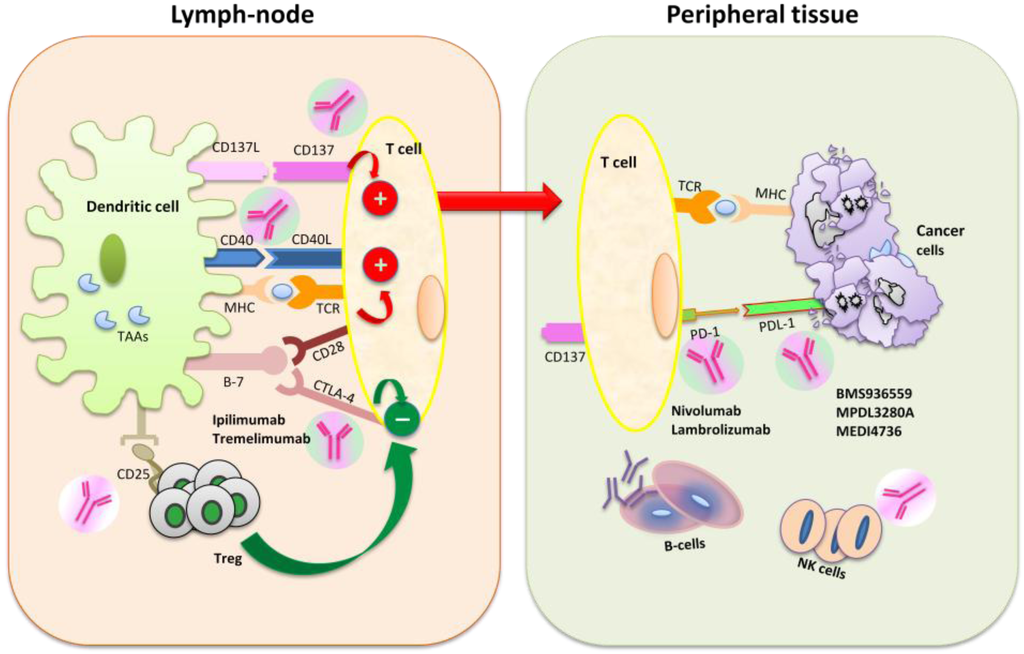
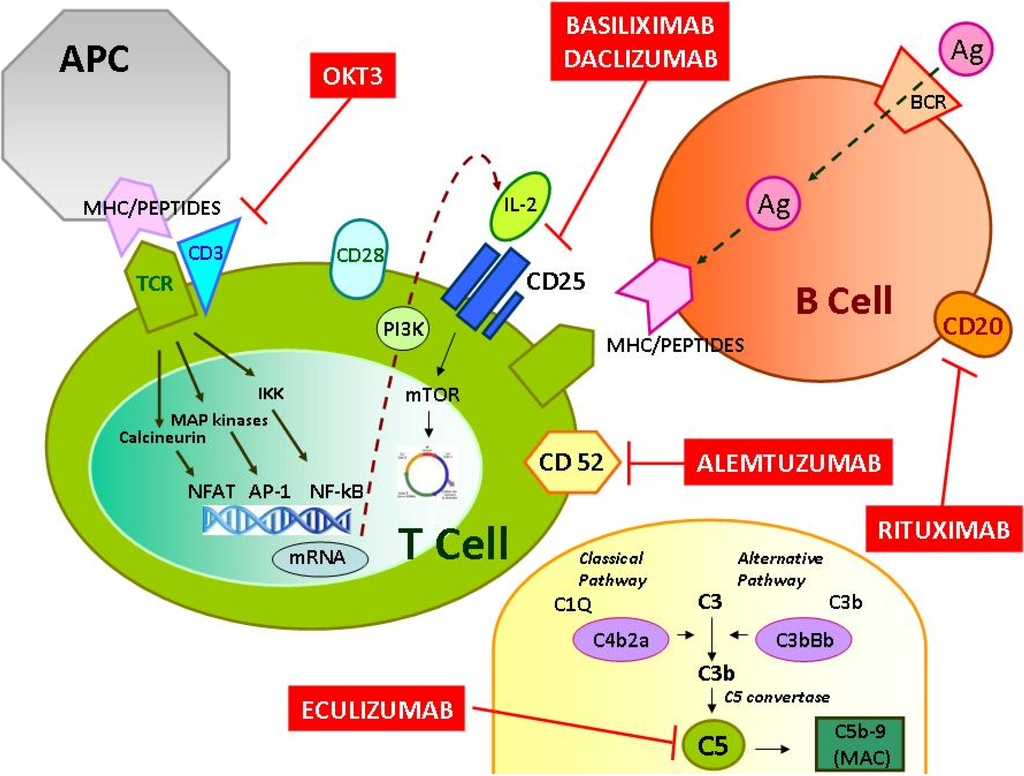
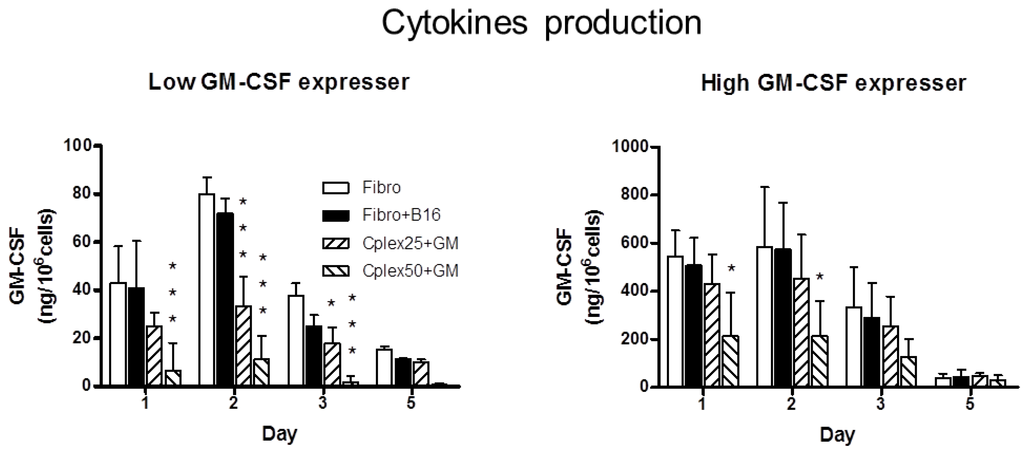
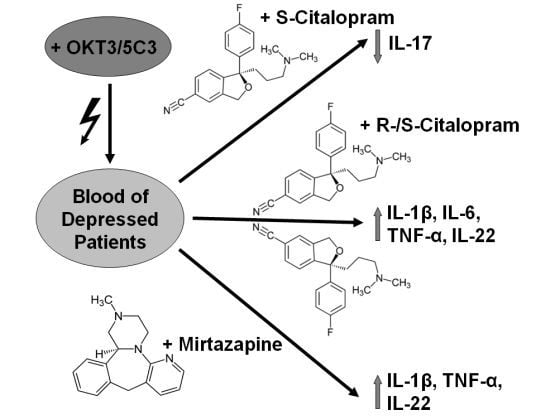
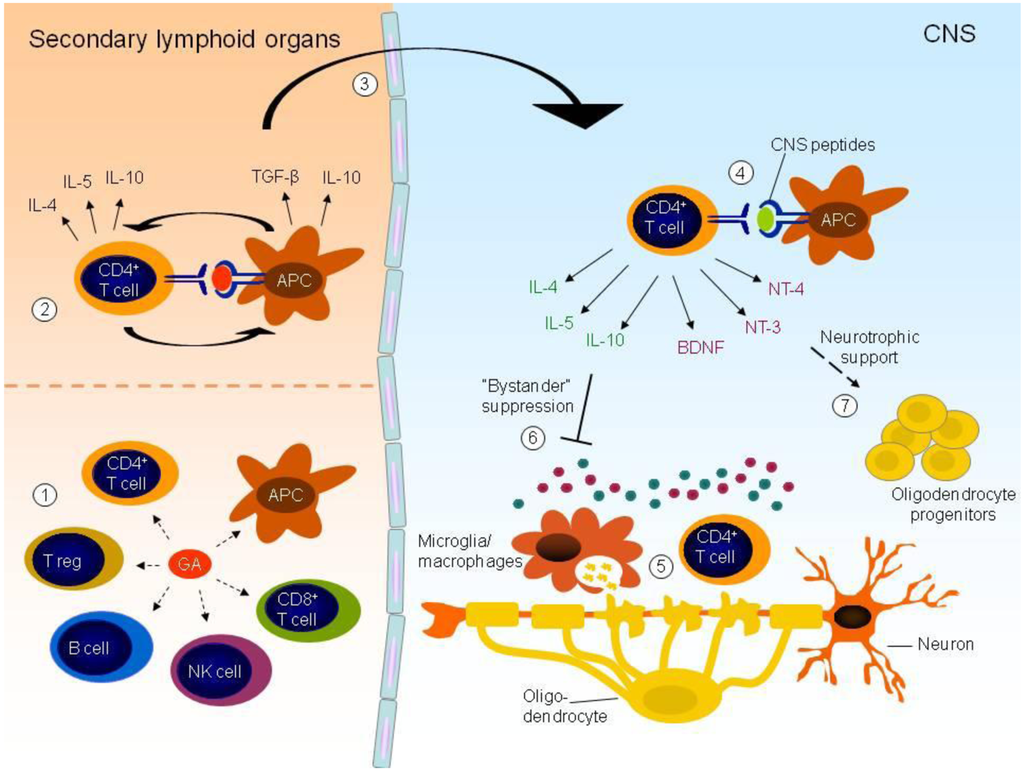
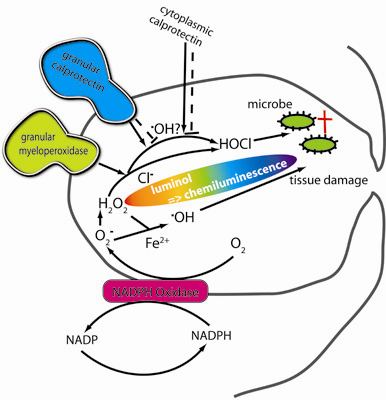
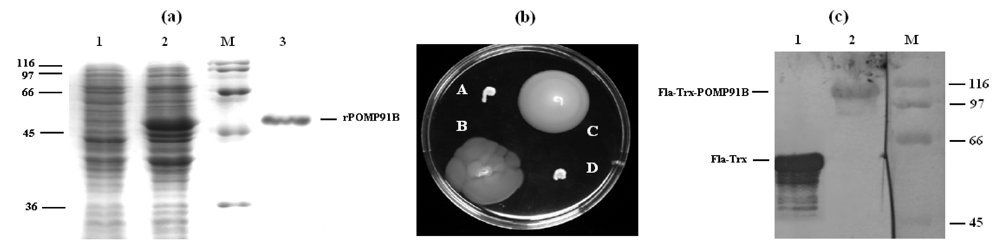
Inflammation and autoimmunity are the major causes of most diseases. They represent one side of the same coin, and are the consequences of deviation the immune system toward detrimental pathways. Diseases such as multiple sclerosis, inflammatory bowel diseases, rheumatoid arthritis, to name few, are manifestations of immune cells attacking normal tissues. On the other side of the coin, low or deficiency of immune cells leads to diseases such as cancer, AIDS, and severe combined immunodeficiency, among many others. Transplantation procedures, particularly those among mismatched individuals require interference with immune cells responsible for tissue rejections and host versus graft diseases. Certain drugs have been developed, whereas others are under evaluation to interfere with immune reactions. This volume of Toxins will deal with all aspects of toxicity that result from over-activity of the immune system. In addition, we will consider papers that deal with therapy of diseases caused by the imbalance of innate and adaptive immune systems. Authors dealing with controlling the toxicity of the immune system as a consequence of therapy, or as a result of genetic or environmental factors are strongly encouraged to submit their papers to this collection of Toxins.
Dr. Azzam A. Maghazachi
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a double-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Toxins is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- toxicity of the immune system
- inflammation
- autoimmune diseases
- transplantation
- cancer
- AIDS
- drugs