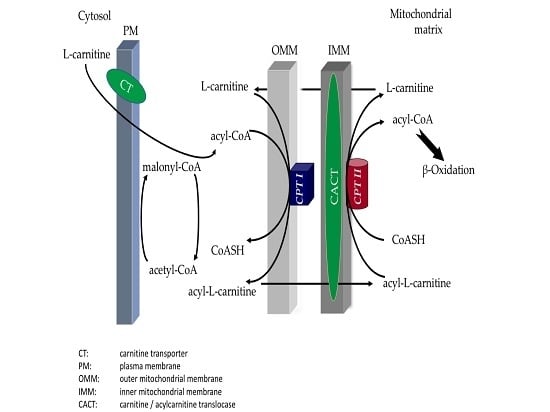
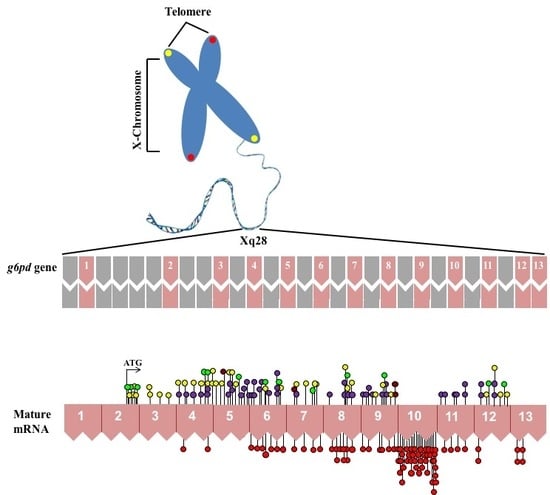
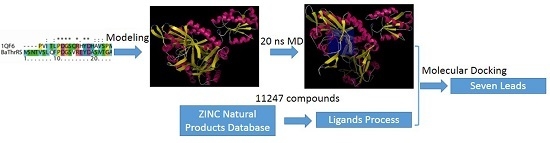
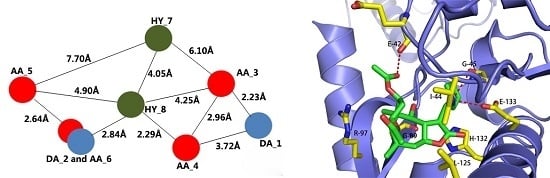
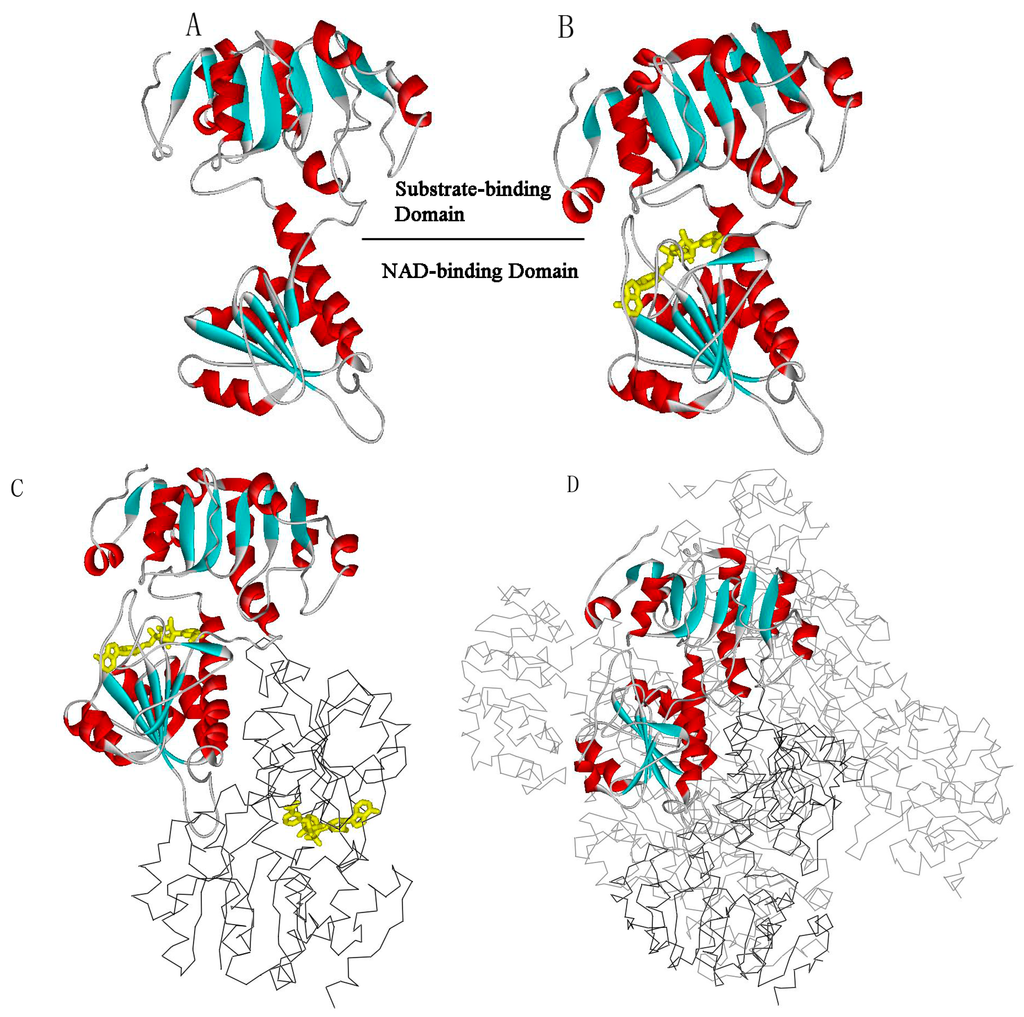
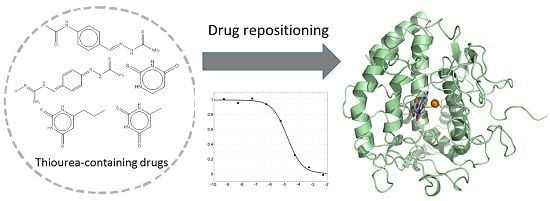
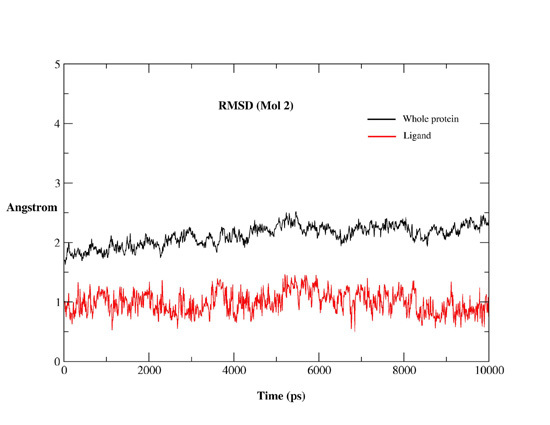
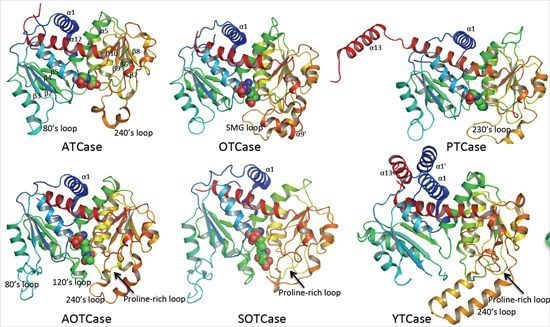
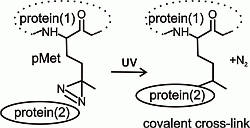
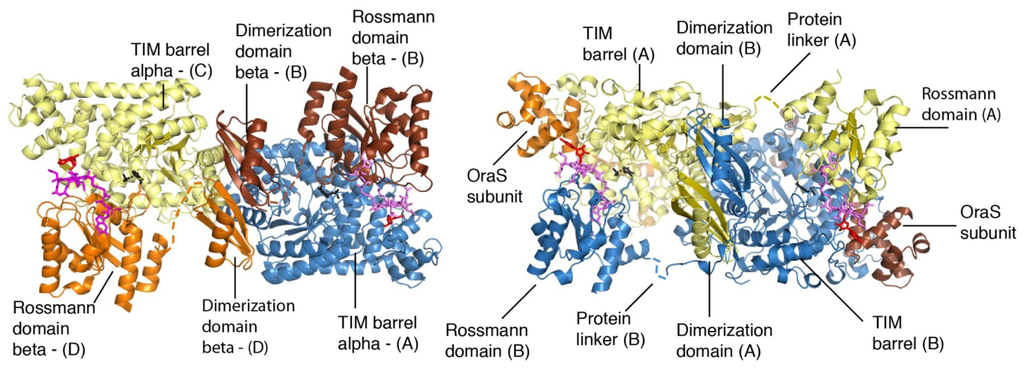
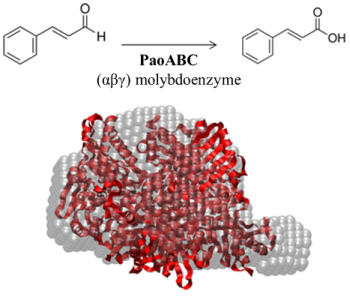
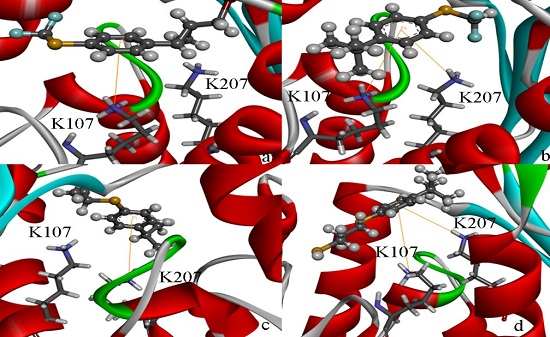
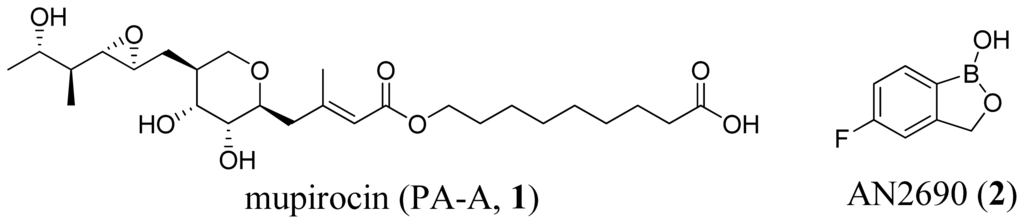
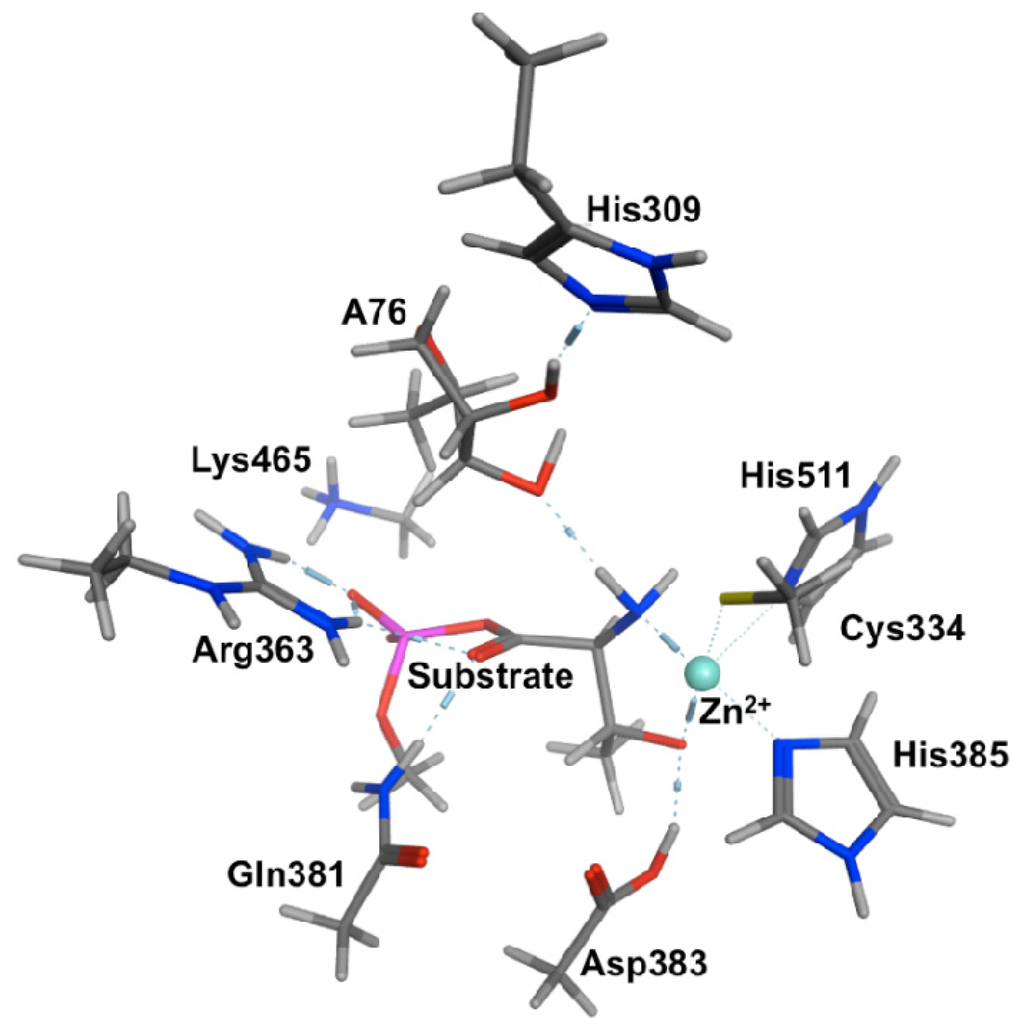
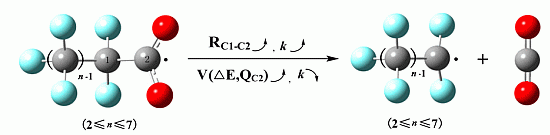Computational, Structural and Spectroscopic Studies of Enzyme Mechanisms, Inhibition and Dynamics
A topical collection in International Journal of Molecular Sciences (ISSN 1422-0067). This collection belongs to the section "Physical Chemistry, Theoretical and Computational Chemistry".
Viewed by 334082Editor
Interests: enzyme reaction mechanisms and dynamics; non-heme iron histone demethylases; multiscale modeling of epigenetic mechanisms
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
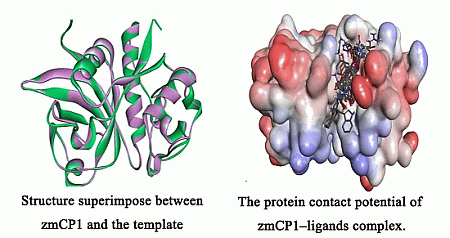
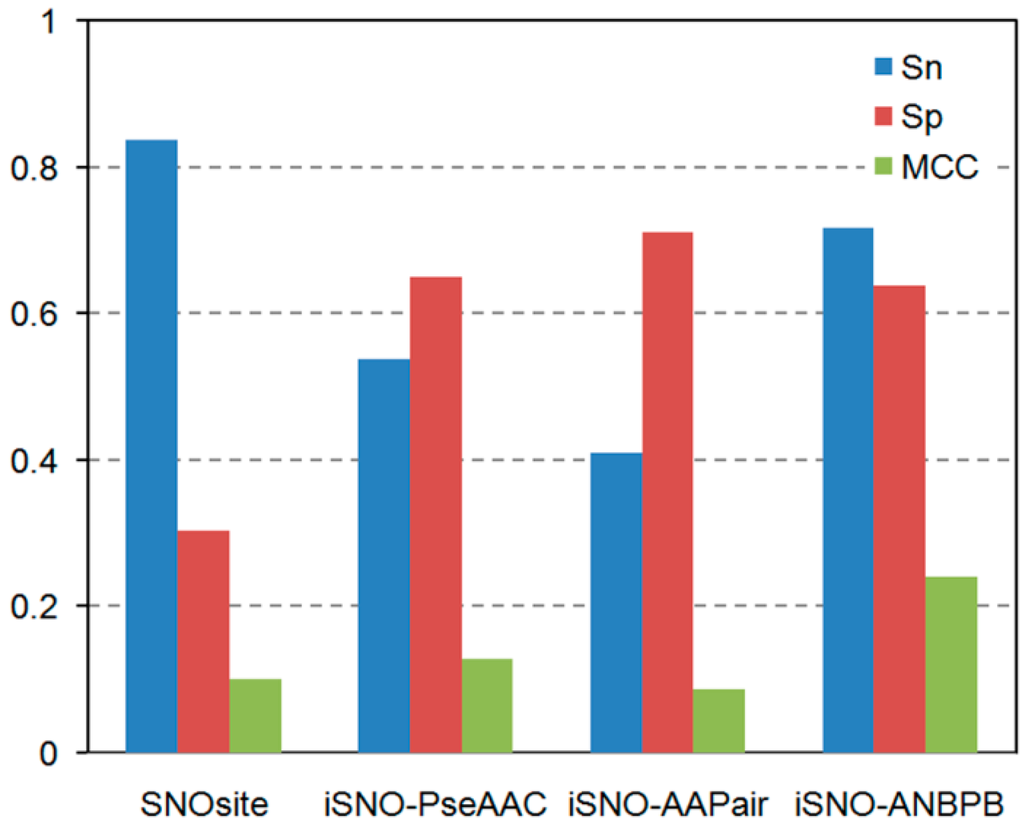
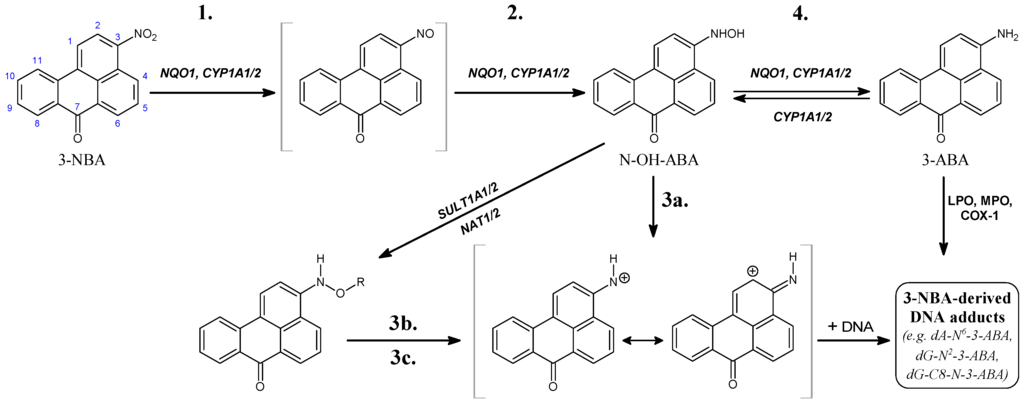
Enzymes are in the center of biochemical processes. They catalyze largest part of all chemical reactions in the living organisms (from viruses to human) and are characterized by unique capabilities to accelerate the reaction rates and to catalyze specific or very selective number of chemical transformations. Not surprisingly the enzymes received massive application in biomedicine, pharmacy, biotechnological and chemical industry.
The current progress in understanding enzymes underlines the new perspective of their applications and utilization in important areas for us. There is vastly growing amount of novel structures, spectroscopic data about intermediates, novel inhibitors synthesized and even enzymes with novel functions engineered.
The current thematic issue of International Journal of Molecular Sciences titled “Computational, Structural and Spectroscopic Studies of Enzyme Mechanisms, Inhibition and Dynamics” is focused on high quality studies by broad range of experimental and computational methods. Contributions focused on integrated modelling/experimental or combination between different experimental methods and the multilevel applications of computational methods are very welcome as well. Highly valued will be combined fundamental and innovative contributions focused on the applications of the enzyme mechanisms and in the all areas with impact for the society: industry, health, food etc. Finally we would like to support strengthen, develop, demonstrate and facilitate the independence of thinking, creativity, initiative of researchers at all levels.
Prof. Dr. Christo Z. Christov
Collection Editor
Manuscript Submission Information
Manuscripts for the topical collection can be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. All papers will be peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on this website. The topical collection considers regular research articles, short communications and review articles. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page.
Please visit the Instructions for Authors page before submitting a manuscript. The article processing charge (APC) for publication in this open access journal is 2900 CHF (Swiss francs).
Keywords
- enzyme mechanisms
- computational modeling
- qm / mm
- molecular dynamics
- enzyme spectroscopy
- quantum mechanics
- enzyme inhibition
- enzyme crystallography