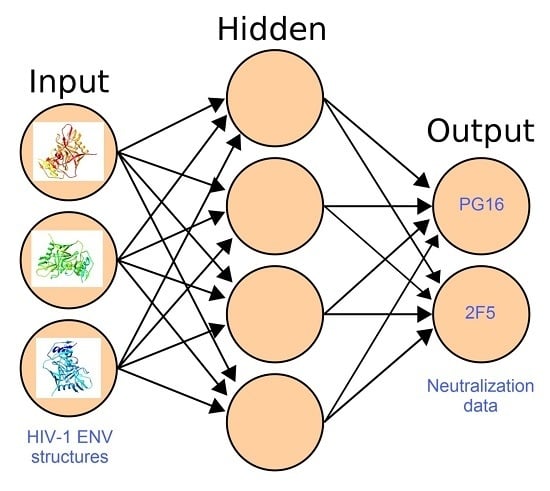
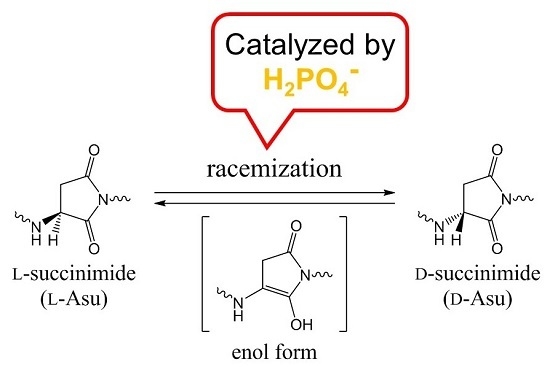
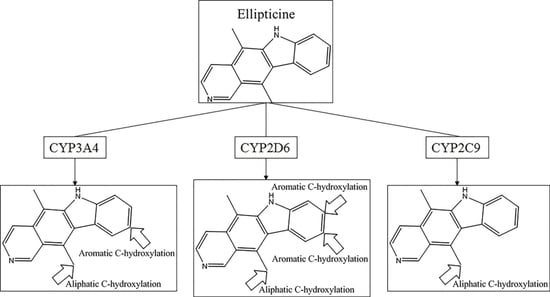
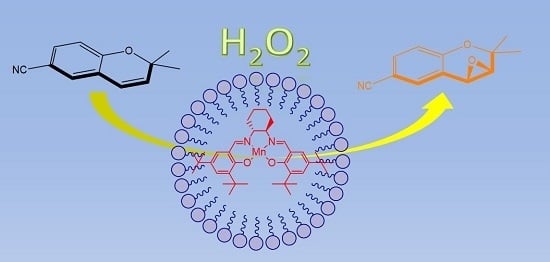
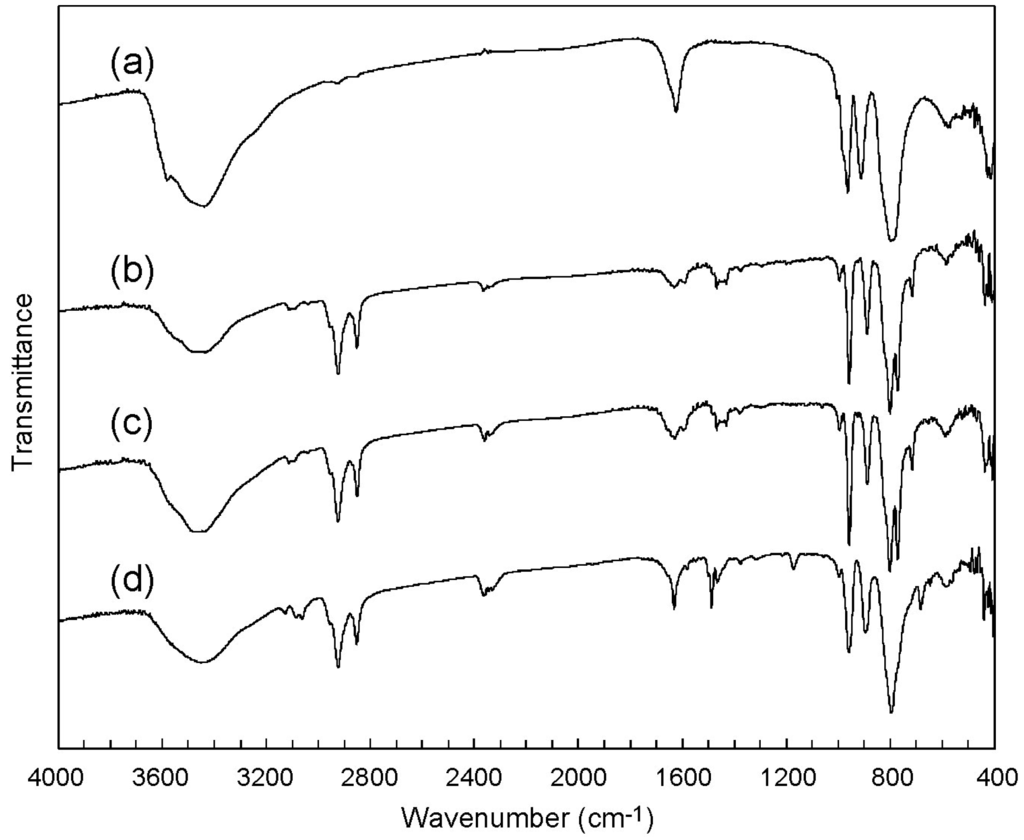
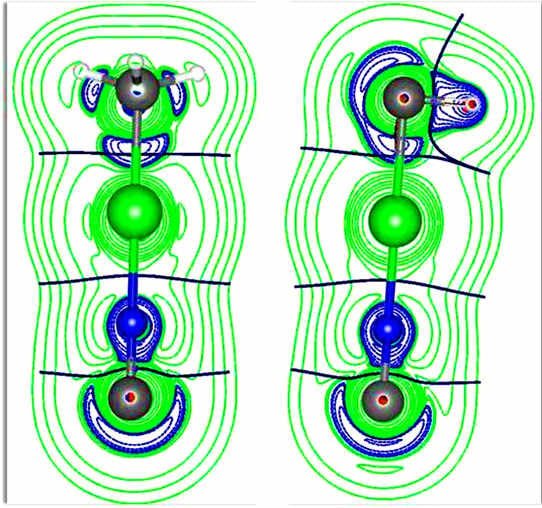
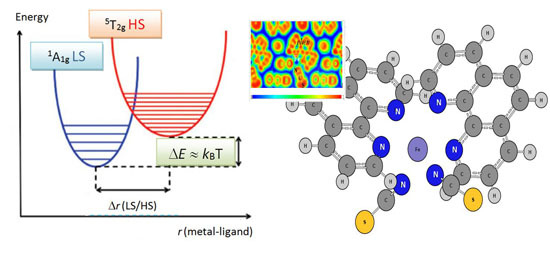
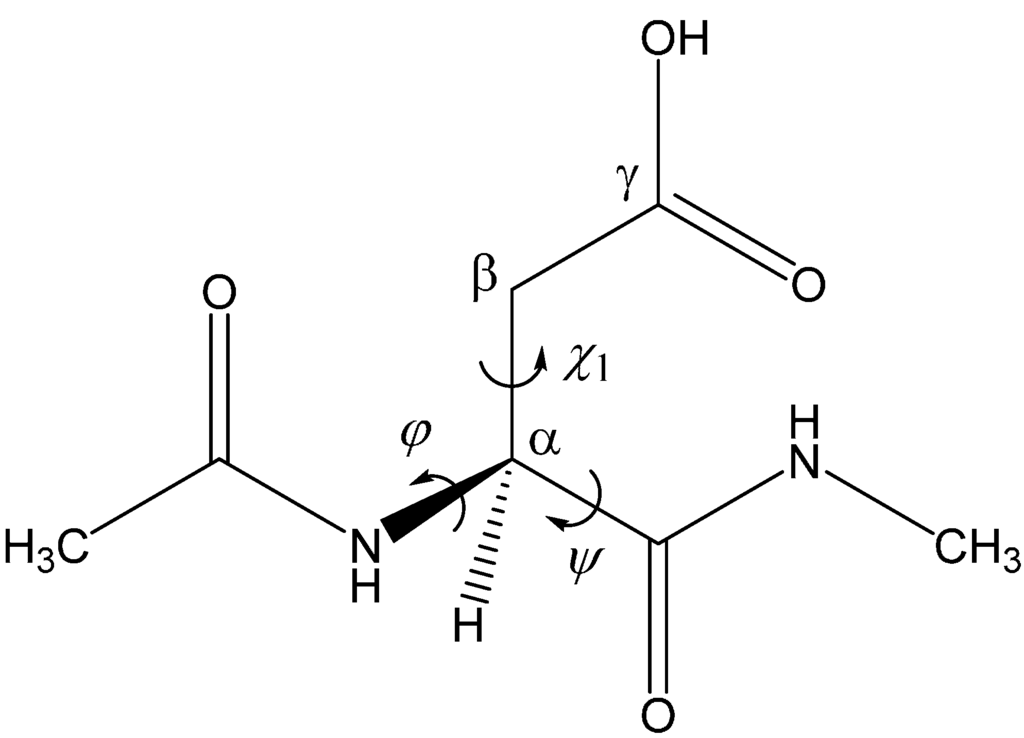
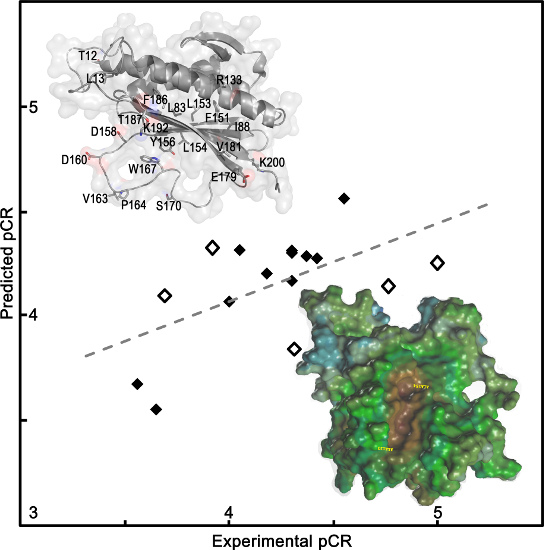
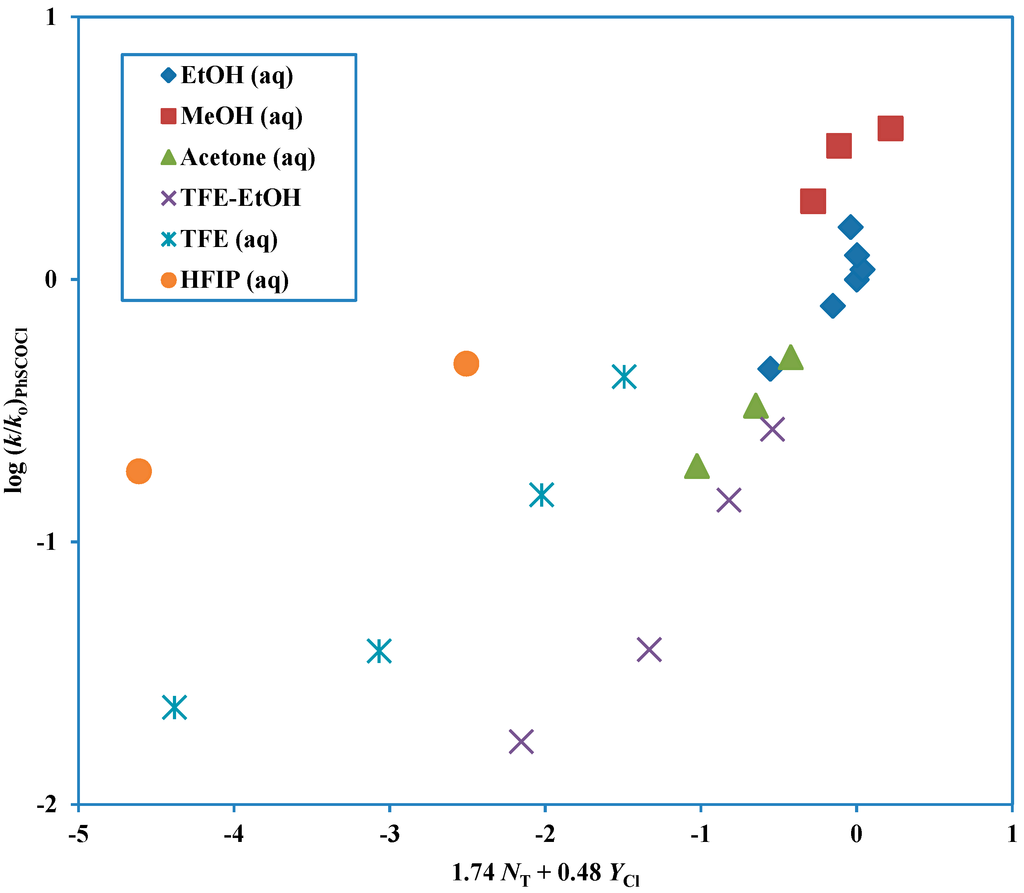
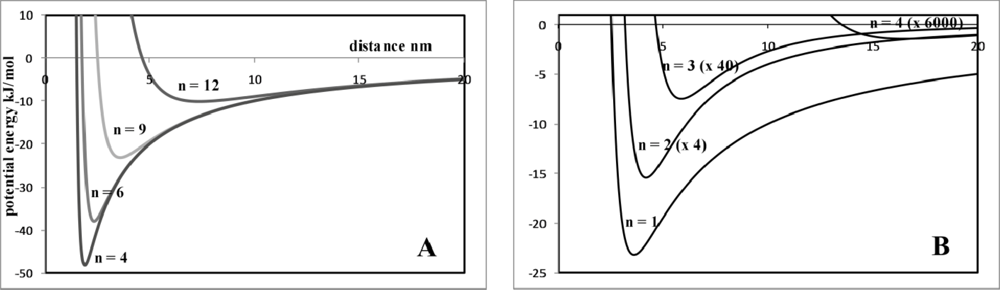
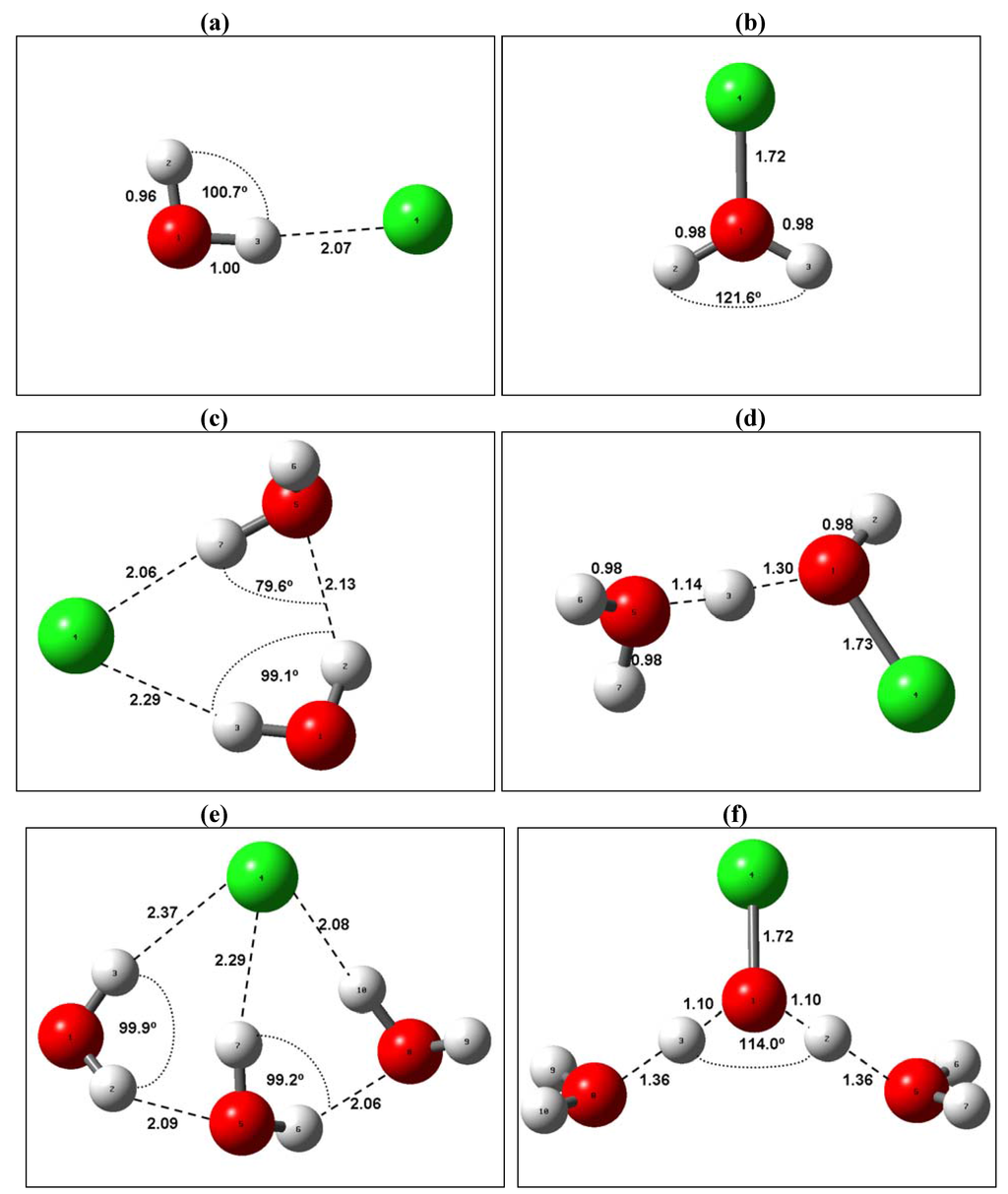
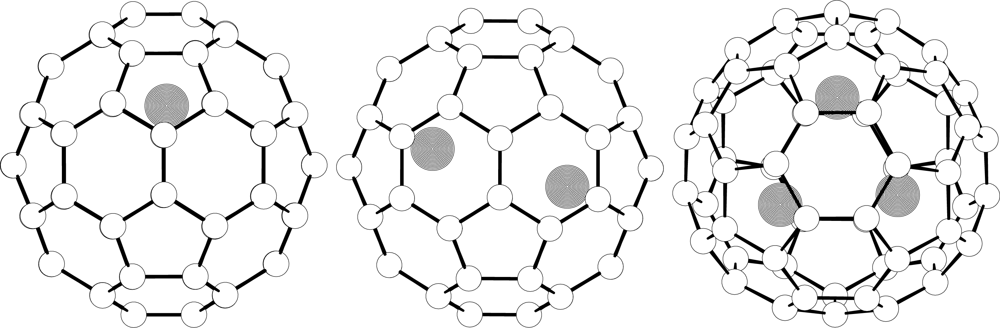
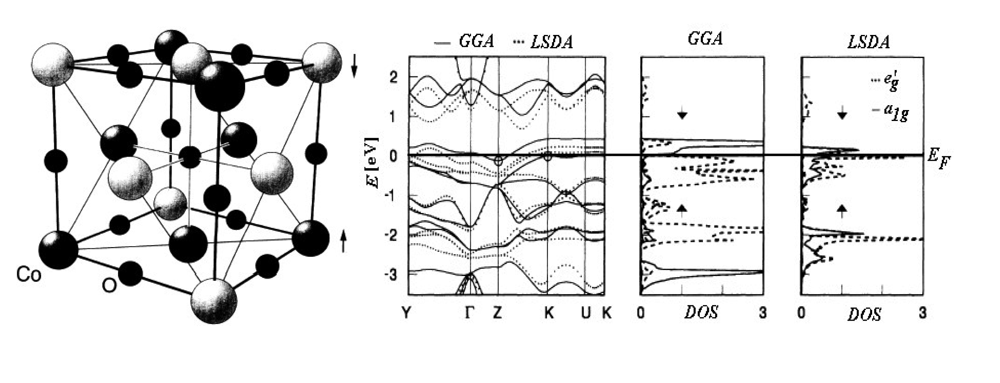
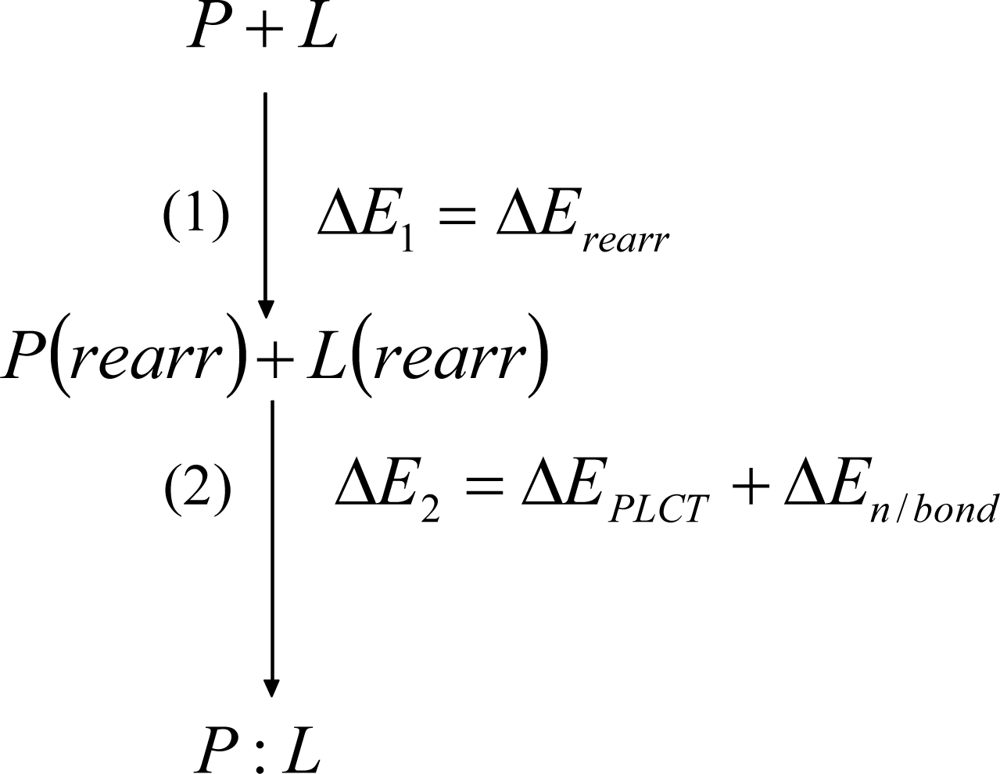Chemical Bond and Bonding
A topical collection in International Journal of Molecular Sciences (ISSN 1422-0067). This collection belongs to the section "Physical Chemistry, Theoretical and Computational Chemistry".
Viewed by 9280Editor
2. Laboratory of Renewable Energies-Photovoltaics, R&D National Institute for Electrochemistry and Condensed Matter–INCEMC–Timisoara, Str. Dr. Aurel Podeanu 144, 300569 Timișoara, Romania
Interests: quantum physical chemistry; nanochemistry; reactivity indices and principles; electronegativity; density functional theory; path integrals; enzyme kinetics; QSAR; epistemology and philosophy of science
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Chemical Bonding is in the core of Chemistry. It actually defines Chemistry as an autonomous science, with a certain objective to study, understand, and develop; it also opens inter-, multi-, and trans-disciplinary junctions with other mathematical (including informatics), natural (physical and quantum) sciences, and with life (bio, eco, medical, pharma) sciences alike. Nevertheless, the fundamental research on unifying the chemical bonds recognized parallels on a different (and non-reductive) level the Great Unification of Forces in Nature as physics advocates; in this line, a foreseen Physicochemical Grand Unification of Forces would equally be a worthy project for humankind increasing knowledge of existence and betterment of life and of its expanding (given the universal nuclei-synthesis or nuclei-genesis, for instance, in cosmology). On the other side of chemical bond applications, various exotic chemical situations have been reported, such as sextupole bonds, nano- and bio-molecules, and carbon-based aggregates that need both conceptual and computational explanations and experimental analysis. The increased need for molecular design for assessing biotargets through pharmacophores, the practical demands of predictions of acute toxicity of medicines, and environmental waste compounds, all these actual realities of Chemistry, in both its principles and applications, deserve a special forum.
In this generous yet challenging context of the reality and manifesting reality of chemical bonding, the dedicated Topical Collection on “Chemical Bond and Bonding” in an open-access molecular-oriented science forum is both an academic, scholarly, and ultimately a social (including knowledge and economic growth by innovation) contribution to the present for future generations of materials and compounds, while having understood and controlled the chemical bond and interactions in nano- to maco-environments.
The Topical Collection on “Chemical Bond and Bonding” has emerged from earlier Special Issues on this vivid subject for Chemistry and allied molecular sciences, and it will continue the dedicated Special Issue as such.
We kindly invite you to contribute papers, expanding on these and allied concepts, for a scientific understanding and control of chemical bonds for a better life and a sustainable environment in the 21st century.
Kind regards,
Dr. Dr-Habil. Mihai V. Putz
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. International Journal of Molecular Sciences is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. There is an Article Processing Charge (APC) for publication in this open access journal. For details about the APC please see here. Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- atoms-in-molecules and chemical epistemology
- bio-chemical and ligand-receptor interaction
- carbon structures and bonding
- chemical bonding and nanochemistry
- chemical graph theory and chemical topology
- electronegativity and chemical reactivity
- computational and quantum chemistry
- crystallography and solid-state chemistry
- sustainable materials
Related Special Issues
- The Chemical Bond and Bonding in International Journal of Molecular Sciences (10 articles - displayed below)
- Chemical Bond and Bonding 2015 in International Journal of Molecular Sciences (21 articles - displayed below)
- Chemical Bond and Bonding 2016 in International Journal of Molecular Sciences (14 articles - displayed below)
- Advances in Chemical Bond and Bonding in International Journal of Molecular Sciences (8 articles)